
ここからコンテンツです。

The World First Asymmetric Synthesis of Halogenated Compounds from Carboxylic Acids
By Kazutaka Shibatomi
A research team at Toyohashi University of Technology has developed a new reaction to produce chlorinated compounds with high isomeric purity. Such compounds are important building blocks for target molecules. However the molecules come in left- and right-handed versions (enantiomers). They can be produced from carboxylic acids, by replacing acid with chlorine. Although conventional methods produce equal mixtures of both isomers、the new method with a chiral amine catalyst specifically yields the desired isomer.
Toyohashi University of Technology researchers led by Associate Professor Shibatomi developed a new catalytic reaction to produce chlorine-containing organic molecules in isomerically pure (left- or right-handed) forms.
Molecules don’t have hands as such, but some of them are left- or right-handed. Many chemical compounds display a feature called chirality, where two versions—known as enantiomers—exist for the same molecule. Although their atoms are connected in exactly the same sequence, the two enantiomers are distinct mirror images, like a pair of hands.
Enantiomers can have very different properties. For example, only the right-handed form of glucose gives you energy—the left-handed isomer cannot be metabolized, even though it tastes the same. Many pharmaceuticals are also chiral, and often only one enantiomer has a medicinal use. Therefore, chemists working on complex molecules have developed a variety of tricks to guarantee isomer purity. However, for some reactions this remains a challenge.
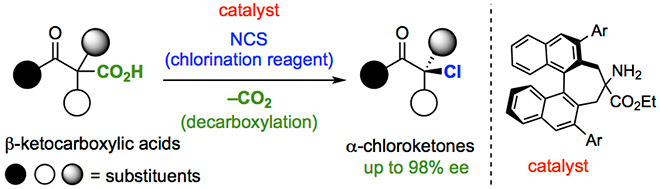
Now, the research team has developed a reaction to produce an important class of compounds in pure left- or right-handed form. Organohalides are molecules in which a halogen, such as chlorine, is bonded to carbon. Many are found in nature, or used in medicine. They can be produced from another family of compounds, carboxylic acids, by simply replacing acid with halogen. Unfortunately, if the target compound is chiral, this substitution produces left- and right-handed isomers in equal amounts.
The research team solved this problem by catalyzing the reaction with a catalyst that is itself chiral. Nowadays, catalysts come in a wide range of shapes and sizes — often rivalling the complexity of the actual target molecule. "We screened a diverse array of chiral catalysts, such as Lewis acid, Brønsted acid, and Lewis base catalysts," study lead author Kazutaka Shibatomi says. "Finally, we found an amine that gave us organohalides with up to 98% enantiomeric purity – even though our starting material was a 50/50 mixture."
The chlorinated products, known as chloroketones, are building blocks for more important chiral molecules like pharmaceuticals. Because chlorine is only weakly bonded to carbon, it can be easily substituted by another atom to make a new molecule. Using one of the many compounds produced in enantiomeric purity by their new reaction, the research team synthesized Cathinone, a natural stimulant.
"The substitution proceeds in a simple, classic way," Associate Prof. Shibatomi says. "While chlorine leaves the molecule on one side, the incoming group approaches from the opposite side. The product’s chirality just depends on the arrangement of these atoms, so if you begin with a pure enantiomer, you retain that purity. This could open up a whole class of compounds that were previously a major challenge to produce as pure enantiomers."
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas ‘Advanced Molecular Transformations by Organocatalysts,’ (26105728) and a Grant-in-Aid for Challenging Exploratory Research (16K13993) from MEXT, Japan. Partial support from Daiichi Sankyo Co., Ltd. and Suzuki Memorial Foundation is also acknowledged. K.K. is grateful to the Leading Graduate School Program R03 of MEXT.
Reference
Kazutaka Shibatomi, Kazumasa Kitahara, Nozomi Sasaki, Yohei Kawasaki, Ikuhide Fujisawa & Seiji Iwasa (2017). Enantioselective decarboxylative chlorination of β-ketocarboxylic acids, Nature Communications,
http://dx.doi.org/10.1038/ncomms15600
世界初、カルボン酸からハロゲン化合物を不斉合成
豊橋技術科学大学の柴富一孝准教授、博士後期課程1年の北原一利さんらの研究グループは、カルボン酸からキラルな塩素化合物を不斉合成することに世界で初めて成功しました。同反応により従来合成が困難であった“キラルクロロケトン”を簡便に高純度で合成することが可能になりました。本研究では生理活性物質合成への応用方法も示されていることから、同手法の医農薬品開発への応用が期待されています。
複雑な構造を有する機能性分子の開発において、これらを精密に合成する技術は不可欠です。中でも医薬品の合成においては、キラル分子の望みとする鏡像異性体のみを高い純度で合成する技術(不斉合成)が必要とされます。生理活性物質の多くは双方の鏡像異性体の薬理活性が異なるため、不要な異性体が混入していると副作用等が懸念されるためです。
キラルハロケトンは医薬品の有用な合成中間体として知られていますが、この化合物を触媒的に不斉合成する手法はほとんど報告されていませんでした。柴富准教授らはカルボン酸をハロゲン原子に変換する脱炭酸的ハロゲン化反応に着目しました。この反応は約150年前に発見された古い反応ですが、これまで同反応の不斉化に成功した例はありませんでした。柴富准教授らは独自に開発した有機分子触媒を利用することでβ−ケトカルボン酸の脱炭酸的塩素化反応が極めて高い不斉収率で進行することを見出しました。本反応では対応するα−クロロケトンが最高98%eeの光学純度で得られます。
得られたクロロケトンの塩素原子は様々な置換基に変換することができます。例えば窒素原子に変換することでアミン化合物が合成でき、硫黄原子に変換すればチオエーテルが合成できます。柴富准教授らは、本手法を利用して強い生理活性を持つ天然有機化合物の合成を達成しました。今後、医薬品の合成ルートの高効率化や新薬開発への応用が期待されています。
本研究は以下の助成を受けて行われました。
- 科研費 新学術領域研究(有機分子触媒による未来型分子変換)No.26105728
- 科研費 挑戦的萌芽研究 No.16K13993
- 博士課程教育リーディングプログラム(超大規模脳情報を高度に技術するブレイン情報アーキテクトの育成)
Researcher Profile

| Name | Kazutaka Shibatomi |
|---|---|
| Affiliation | Department of Environmental and Life Sciences |
| Title | Associate Professor |
| Fields of Research | Synthetic Organic Chemistry / Asymmetric Synthesis / Pharmaceutical Chemistry |
ここでコンテンツ終わりです。
