
ここからコンテンツです。

Challenges for the development of oxide-based all-solid-state batteries
Thick-film cathode solidified on garnet-type oxide solid electrolyte at room temperatureBy Ryoji Inada
A research group led by Associate Professor Ryoji Inada have successfully fabricated a lithium trivanadate (LVO) cathode thick film on a garnet-type oxide solid electrolyte using the aerosol deposition method. The LVO cathode thick-film fabricated on the solid electrolyte performed well, achieving a capacity of up to 300 mAh/g for both reversible charge and discharge, as well as a good cycling stability at 100 ºC. This finding may contribute to the realization of highly safe and chemically stable oxide-based all-solid-state lithium batteries.
Rechargeable lithium-ion batteries (LiBs) have been widely utilized globally as a power source for mobile electronic devices such as smart phones, tablets, and laptop computers because of their high-energy density and good cycling performance. Recently, the development of middle- and large-scale LiBs has been accelerated for use in automotive propulsion and stationary load-leveling for intermittent power generation from solar or wind energy. However, a larger battery size causes more serious safety issues in LIBs; one of the main reasons is the increased amount of flammable organic liquid electrolytes. The research results were reported in Materials on September 1st, 2018.
All-solid-state LiBs with nonflammable inorganic Li-ion (Li+) conductors as solid electrolytes (SE) are expected to be the next generation of energy storage devices because of their high energy density, safety, and reliability. The SE materials must have not only high lithium-ion conductivity at room temperature, but also deformability and chemical stability. Oxide-based SE materials have a relatively low conductivity and poor deformability compared to sulfide-based ones; however, they have other advantages such as chemical stability and ease of handling.
The garnet-type fast Li+ conducting oxide, Li7-xLa3Zr2-xTaxO12 (x = 0.4-0.5, LLZTO), is considered as a good candidate for SE because of its good ionic conductiivity and high electrochemical stability. However, high-temperature sintering at 1000-1200 ºC is generally needed for densification, and this temperature is too high to suppress undesired side effects at the interface between the SE and the majority of electrode materials. Therefore, there are currently few options when it comes to electrode materials that can be used for solid-state batteries with garnet-type SEs developed by the co-sintering process.
Ryoji Inada and his colleagues at the Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, succeeded in fabricating a lithium trivanadate (LiV3O8, LVO) thick-film cathode on garnet-type LLZTO by means of the aerosol deposition (AD) method. All-solid-state cell samples were prepared and tested using the fabricated composite.
The AD method is known to be a room-temperature film-fabrication process, which uses the impact-consolidation of ceramic particles onto a substrate. By controlling the particle size and morphology, dense ceramic thick films can be fabricated on various substrates without thermal treatment. This feature is attractive in the fabrication of oxide-based solid-state batteries because various electrode active materials can be selected and formed on SE without the need for thermal treatment.
LVO has been studied at length as a cathode material for Li-based batteries because of its large Li+ storage capacity of approximately 300 mAh/g. However, the feasibility of LVO as a cathode for solid-state batteries has not yet been investigated. The reaction of LVO initiates at the discharging (i.e., Li+ insertion) process, which differs from that of other conventional cathode materials of LiBs such as LiCoO2, LiMn2O4, and LiFePO4. Therefore, graphite anodes, which are widely used in current LiBs, are difficult to use in batteries with LVO cathodes. In solid-state batteries with garnet-type SEs, Li metal electrodes may potentially be used as anodes; thus, LVO would become an attractive candidate for high-capacity cathodes.
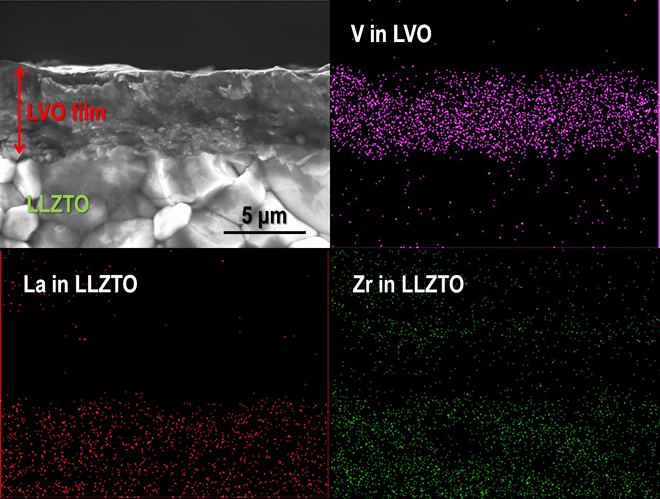
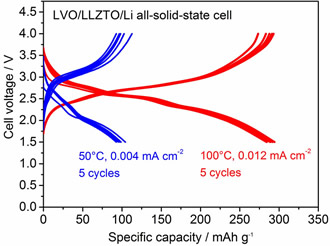
To fabricate a dense LVO film on an LLZTO pellet, the size of the LVO particles was controlled by ball-milling. As a result, an LVO thick film with a thickness of 5-6 µm was successfully fabricated on LLZTO at room temperature. The relative density of the LVO thick film was approximately 85%. For the electrochemical characterization of the LVO thick film as a cathode, Li metal foil was attached on the opposite end face of the LLZTO pellet as an anode to form an LVO/LLZTO/Li structured solid-state cell. The galvanostatic charge (Li+ extraction from LVO) and discharge (Li+ insertion into LVO) properties in an LVO/LLZTO/Li all-solid-state cell were measured at 50 and 100 ºC.
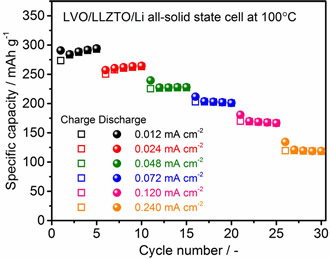
Although the polarization was considerably high at 50 ºC, a reversible capacity of approximately 100 mAh/g was confirmed. With an increase in temperature to 100 ºC, the polarization reduced and the capacity increased significantly to 300 mAh/g at an averaged cell voltage of approximately 2.5 V; this is a typical behavior of an LVO electrode observed in an organic liquid electrolyte. In addition, we confirm that the charge and discharge reactions in the solid-state cell are stably cycled at various current densities. This can be attributed to the strong adhesion between the LVO film fabricated via impact consolidation and the LLZTO and LVO particles in the film.
These results indicate that LVO can potentially be used as a high-capacity cathode in an oxide-based solid-state battery with high safety and chemical stability, even though additional investigation is needed to enhance the performance. Researchers have carried out further studies to realize oxide-based solid-state batteries at lower operating temperatures.
This work was partly supported by Grant-in-Aids for Scientific Research (C) (Grant No. 16K06218) and Fund for the Promotion of Joint International Research (Fostering Joint International Research) (Grant No. 16KK0127) from the Japan Society for the Promotion of Science (JSPS).
Reference
Ryoji Inada, Kohei Okuno, Shunsuke Kito, Tomohiro Tojo and Yoji Sakurai (2018). Properties of Lithium Trivanadate Film Electrode Formed on Garnet-type Oxide Solid Electrolyte by Aerosol Deposition, Materials 11(9), 1570, 2018.
https://doi.org/10.3390/ma11091570
酸化物を用いて超安全な電池の実現をめざす
常温で固めた酸化物全固体リチウム電池用正極の特性By 稲田 亮史
豊橋技術科学大学の稲田准教授らの研究グループは、エアロゾル・デポジション(AD)法を用いてガーネット型酸化物固体電解質上にバナジウム酸リチウム(LVO)正極を常温で作製することに成功しました。対極を金属リチウムとして構成した全固体電池を100ºCで動作した結果、300 mAh/g(LVO単位重量当たり)の充放電容量を確認しました。本成果は、高い安全性と化学的安定性を備えた酸化物全固体リチウム電池の実現に役立つものです。
現在、リチウムイオン電池はスマートフォンやタブレット、ノートパソコン等の小型電子機器用電源として世界中で使われています。最近では、プラグイン・ハイブリット車や電気自動車用車載電源や太陽光発電や風力発電で得られる電力を貯蔵する蓄電システム等への応用も進んでいます。電池の大型化に向けて、その安全性の確保は非常に大切ですが、現行のリチウムイオン二次電池には燃えやすい電解液が使われており、電池内で異常が発生した際に破裂や発火を引き起こす原因となります。
燃えやすい電解液の代わりに燃えない固体電解質(固体のリチウムイオン伝導体)を用いた全固体リチウムイオン電池は、高いエネルギー密度と高安全性・信頼性を同時に達成できる蓄電デバイスとして期待されています。固体電解質として使われる材料には、高いイオン伝導率の他に、成型性や化学的安定性といった多くの要件が求められます。酸化物系固体電解質は硫化物系固体電解質と比較してイオン伝導率や成形性は劣りますが、化学的安定性や取扱いの簡単さといった利点があります。
ガーネット型結晶構造を持つリチウム含有酸化物Li7-xLa3Zr2-xTaxO12(x = 0.4-0.5, LLZTO)は、優れたイオン伝導特性と電気化学的安定性を示すため、全固体電池用固体電解質として有望視されています。本材料の高密度化には一般に1000-1200ºCでの焼結が必要ですが、電極材料と接合した際の副反応を防ぐにはこの温度は高過ぎます。これが一因で、電極と固体電解質間の接合に有効な共焼結プロセスが使える電極材料は限られています。
豊橋技術科学大学電気・電子情報工学系の稲田亮史准教授と同准教授の研究グループのメンバーは、高容量正極材料として知られているバナジウム酸リチウム(LiV3O8, LVO)を、常温成膜プロセスの一つであるエアロゾル・デポジション(AD)法を利用して固体電解質上で電極化することに成功しました。また、作製した試料を用いて全固体リチウム電池を試作し、その電気化学特性を評価しました。
AD法はセラミックス粒子の常温下での衝撃固化現象を利用した成膜技術です。原料となるセラミックス粒子の形状やサイズをうまく制御すれば、高密度な厚膜を熱アシストなしで様々な基板の上に作ることができます。この特徴により、様々な電極材料と固体電解質材料の一体化が可能になるので、酸化物全固体電池を作製する上で魅力的なプロセスです。
LVOは大きな充放電容量を示すリチウム二次電池用正極材料として古くから研究されてきましたが、全固体電池用正極としての研究例はありませんでした。また、現行のリチウムイオン電池で使われているLiCoO2やLiMn2O4, LiFePO4といった正極材料とは違って充電状態で作製されるため、現行電池に使われている黒鉛を負極とする電池にLVO正極をそのまま使うのは困難です。ガーネット型固体電解質を用いた全固体電池では金属リチウムを負極に使える可能性があり、LVOは魅力的な正極材料の候補となり得ると考えられます。
同研究グループは、ボールミル処理により粒子サイズを調整したLVO粉末を用いて、LLZTO上にLVO厚膜(厚さ5-6 µm、相対密度85%)を常温で固化することに成功しました。作製したLVO電極の特性を評価するために、金属リチウムをLLZTOの片端面に圧接し、LVO/LLZTO/Li積層構造を持つ全固体リチウム電池を試作し、50ºCおよび100ºCにおける充放電試験を行いました。
50ºCでは過電圧がかなり大きいものの、約100 mAh/gの可逆容量が得られました。測定温度を100ºCに上げると、過電圧の低下と充放電容量の増加が見られ、300 mAh/g程度の大きな可逆容量とLVOに特有な充放電曲線が得られました。更に、電流密度を0.012-0.240 mA/cm2(LVOあたり15-300 mA/gに相当)の範囲で変えた条件においても、良好なサイクル安定性を確認できました。以上の結果は、LVO厚膜と固体電解質間、および厚膜内のLVO粒子間の結着力が強いためと考えられます。
本研究の成果は、LVOが酸化物全固体電池用高容量正極として適用できる可能性を示すものですが、その一方で、電池性能の向上に向けては更なる研究が必要不可欠です。同研究グループでは、酸化物系全固体電池の低温動作に向けた様々な検討を現在進めています。
本研究の一部は日本学術振興会(JSPS)科学研究費助成事業(基盤研究(C)16K06218および国際共同研究加速基金(国際共同研究強化)16KK0127)の支援の下で実施されたものです。
Researcher Profile

| Name | Ryoji Inada |
|---|---|
| Affiliation | Department of Electrical and Electronic Information Engineering |
| Title | Associate Professor |
| Fields of Research | Energy Conversion Engineering / Electrical and Electronic Material Engineering / Non-Destructive Testing |
ここでコンテンツ終わりです。
