Numano, Rika
| Affiliation | Institute for Research on Next-generation Semiconductor and Sensing Science (IRES²) |
|---|---|
| Concurrent post | Center for Diversity and Inclusion Department of Applied chemistry and Life Science |
| Title | Professor |
| Fields of Research | chronobiology, molecular biology, neuroscience |
| Degree | Ph.D. |
| Academic Societies | The Molecular Biology Society of Japan, Japanese Society for Chronobiology,The Japan Neuroscience Society, European Biological Rhythm Society |
| numano@ Please append "tut.jp" to the end of the address above. |
|
| Laboratory website URL | https://www.eiiris.tut.ac.jp/numano/ |
| Researcher information URL(researchmap) | Researcher information |
Theme1:Monitoring mammalian circadian rhythms using Per1:luc Tg mice
Overview
The biochemical, physiological and behavioral processes are under the control of internal clocks with the period of approximately 24 hr, circadian rhythms. The expression of mouse Period1 (mPer1) gene oscillates autonomously in the suprachiasmatic nucleus (SCN) and is induced immediately after a light pulse. Per1 is an indispensable member of the central clock system to maintain the autonomous oscillator and syncholonize envilonmental light cycle.
I constructed Per1:luc Tg mice and rats in which firefly luciferase was rhythmically expressed under the control of the mouse Per1 promoter in order to monitor mammalian circadian rhythms by Per1 rhythmic expression. Rhythmic emission from the cultured SCN slices of Tg persisted for up to some months in vitro, while those from peripheral tissues like liver also were found but damped after two to seven cycles in vitro. It suggests that a self-sustained circadian pacemaker in the SCN entrains circadian oscillators in the periphery to each adaptive phase.
I am performing functional analysis of circadian pacemaker neurons in the SCN by TOYOHASHI original electrophysiological probe.
Selected publications and works
1Resetting central and peripheral circadian oscillators in transgenic rats.
*Yamazaki S, * Numano R, *Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H.
Science, 288, 2000, p682-685 *equally contributed
2Constitutive expression of the Period1 gene impairs behavioral and molecular circadian rhythms.
Numano R, Yamazaki S, Umeda N, Samura T, Sujino M, Takahashi R, Ueda M, Mori A, Yamada K, Sakaki Y, Inouye ST, Menaker M, Tei H.
Proc Natl Acad Sci U S A, 103, 2006, p3716-3721
3Nanoscale tipped microwire arrays enhance electrical trap and depth injection of nanoparticles.
Goryu A, Numano R, Ikedo A,Ishida M, Kawano T.,Nanotechnology Vol.23,41,2012
4In vivo bioluminescence and reflectance imaging of multiple organs in bioluminescence reporter mice by bundled-fiber-coupled microscopy
Ando Y, i Sakurai T, Koida K, Tei H, Hida A, Nakao K, Natsume M, and Numano R,
Biol.I Optics Eexp., 7(3), 963-978,2016
Keywords
Theme2:Manipulation of neural activity under optical control by bionanomachine.
Overview
I recognize intact biostructure ionotropic glutamate receptors (iGluR6) as machinery, which is normally expressed in synaptic neural processes in mammalian brain. To control any neural activity remotely and reversibly, photoswitchable nanomachine?LiGluR?were developed based on iGluR6 and operated using photoiosomerizable new chemicals, MAG. Two iGluR6 mutants could be photo-switched using a series of maleimde-azobenzene-glutamate (MAG) compounds, which dangled 2R,4R-allyi glutamate (G) from a linker containing the photoisomerizable azobenzene (A) that was attached to the introduced cysteines via maleimide (M). Three kinds of MAGs with different linker lengths (shortest :MAG0, middle: MAG1, longest: MAG2) were examined at 16 cysteine positions around the “mouth” of the ligand binding domain “clamshell” from geometry. LiGluR(with L439C mutation): opening in UV light and closing in visible light by all MAGs. In neural cells with LigluR, action potentials were optimally evoked and extinguished by UV and visible light, respectively. These photo-switched nanomachines could manipulate neural activity under optical control both in vitro and in vivo. The researchers could control neural activity to regulate physiology from a mechanistic view.
Selected publications and works
5Allosteric control of an ionotropic glutamate receptor with an optical switch.
Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D.
Nat Chem Biol., 2, 2006, p47-52
6Remote control of neuronal activity with a light-gated glutamate receptor.
Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, Kramer RH, Flannery J, Baier H, Trauner D, Isacoff EY.
Neuron, 54, 2007, p535-545
7Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor.
Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY.
Proc Natl Acad Sci U S A, 104, 2007,p10865-10870
8Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR.
Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, Roux B, Trauner D, Isacoff EY.
Proc Natl Acad Sci U S A, 106, 2009,p6814-6819
Keywords
Theme3:New innovative method for delivering genes into cells
Overview
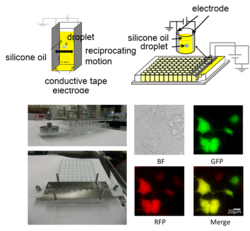
Researchers from Toyohashi Tech have developed a novel transfection method using water-in-oil droplet electroporation: a liquid droplet with exogenous DNA and cells that is suspended between electrodes in dielectric oil is exposed to a DC electric field. This technique can contribute to biomedical innovation in high-throughput screening.
The authors, Assistant Professor Hirofumi Kurita and Associate Professor Rika Numano, said, "This technique will lead to the development of cell transfection in regenerative medicine and gene therapy."
Selected publications and works
Novel Parallelized Electroporation by Electrostatic Manipulation of a Water-in-Oil Droplet as a Microreactor.
Kurita H, Takahashi S, Asada A, Matsuo M, Kishikawa K, Mizuno A, Numano R.
PLoS One. 2015 Dec 9;10(12):e0144254. doi: 10.1371/journal.pone.0144254. eCollection 2015.
Keywords
Others (Awards, Committees, Board members)
Award of the soroptimist Japan Fundation of TOYOHASHI 2013