Yoshida, Eri
| Affiliation | Department of Applied chemistry and Life Science |
|---|---|
| Title | Associate Professor |
| Fields of Research | Ecomaterial Engineering / Supramolecular Chemistry / Polymer Chemistry |
| Degree | PhD in Engineering (Tokyo Institute of Technology) |
| Academic Societies | American Chemical Society / Royal Society of Chemistry / The Chemical Society of Japan / The Society of Polymer Science, Japan / The Society of Chemical Engineers, Japan / Japan Oil Chemists / |
| yoshida.eri.gu Please append "tut.jp" to the end of the address above. |
|
| Laboratory website URL | https://envmater.chem.tut.ac.jp/Eri-Lab/Home.html |
| Researcher information URL(researchmap) | Researcher information |
Research
(1)Synthesis of Advanced Materials from Carbonic AcidYoshida's lab has been creating advanced nanomaterials from carbonic acid.
(2)Waste Plastics Depolymerization
This laboratory has been developing efficient methods for the depolymerization of waste plastics.
(3)Creation of Artificial Biomembrane Models Using Synthetic Polymers
The laboratory has been fabricating artificial biomembrane models using synthetic polymers prepared through photo-controlled/living radical polymerization.
Theme1:Synthesis of Advanced Materials from Carbonic Acid
Overview
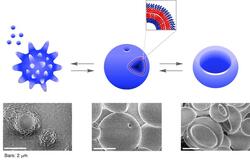
To reduce global warming caused by increased CO2 emissions, the Yoshida Lab has been creating various advanced materials using carbonic acid in aqueous systems. The method produces electrolytes that can reversibly and repeatedly capture carbonic acid simply by introducing CO2 into an amine solution. It also yields ribbon-like polymer complexes that store carbonic acid within their structures, and spherical or rod-like nanocapsules that feature carbonic acid on their shells through the self-assembly of carbonic acid-captured polyelectrolytes. The use of carbonic acid in material design contributes not only to reducing CO2 emissions from industries but also to preserving marine ecosystems from ocean acidification.
Selected publications and works
1. E. Yoshida, Utilization of CO2-captured poly(allylamine) as a polymer surfactant for nanoarchitecture production in a closed CO2 cycle, RSC Sustainability, 2024, 2, 1837–1848, https://doi.org/10.1039/D4SU00121D
2. E. Yoshida, CO2 capture-induced polymer complexes. Carbon Capture Sci. Technol. 2022, 2, 100038, 1-7. https://doi.org/10.1016/j.ccst.2022.100038
3. E. Yoshida, CO2 capture-induced electrolytes using tertiary diamines. SCIREA J. Chem. 2020, 5(2), 12-29. http://www.scirea.org/journal/PaperInformation?PaperID=2980
4. E. Yoshida, CO2-responsive behavior of polymer giant vesicles supporting hindered amine. Colloid Polym. Sci. 2019, 297, 661-666. https://doi.org/10.1007/s00396-019-04484-8
Keywords
Theme2:Waste Plastics Depolymerization
Overview
To address the intensifying issue of plastic waste, establishing closed-loop recycling systems is crucial. These systems involve recovering component monomers from waste plastics and utilizing them to reproduce the original products. The Yoshida Lab has established a convenient chemical recycling method for waste polystyrene foam using vacuum pyrolysis depolymerization and a spirit lamp flame, recovering high-purity monomer. Applicable to all types of non-biodegradable waste plastics with carbon-carbon backbones, this method shows promise in reducing pollution caused by waste plastics. By returning waste plastics to their original fossil fuel state without the need for further fractionation and purification, it demonstrates the potential for waste plastics to serve as an alternative source for petroleum production, thereby reducing natural fossil fuel consumption.
Selected publications and works
1. Eri Yoshida, High-purity monomer recovery from commercial engineering plastics by vacuum pyrolysis depolymerization, RSC Sustain. 2024, 2, 3909 – 3915. DOI: 10.1039/D4SU00614C.
2. E. Yoshida, Vacuum pyrolysis depolymerization of waste polystyrene foam into high-purity styrene using spirit lamp flame for convenient chemical recycling, RSC Sustainability, 2023, 1, 2058–2065. https://doi.org/10.1039/d3su00207a
Keywords
Theme3:Creation of Artificial Biomembrane Models Using Synthetic Polymers
Overview
Micron-sized giant vesicles are viable artificial models for biomembranes of cells and organelles, based on their similarities in size and structure. The Yoshida Lab has fabricated artificial biomembrane models using synthetic polymer vesicles. These were prepared by the polymerization-induced self-assembly method, utilizing the nitroxide-mediated photo-controlled/living radical polymerization technique developed by this lab. The polymer vesicles can replicate static and dynamic morphologies of biomembranes, including villus-like structures for the digestive system, anastomosed tubular networks with fenestrated sheets for the endoplasmic reticulum and Golgi apparatus, perforated vesicles for the nuclear envelope, cup-shaped vesicles for the isolation membrane formed during the early stages of autophagy, neuron-like tubular extensions, and human erythrocyte-like morphology transformations. These findings contribute to understanding the differences between inanimate and animate matter, promising insights into the origin of life and the fabrication of artificial life using non-natural synthetic inanimate materials.
Selected publications and works
1. E. Yoshida, Mechanisms of cup-shaped vesicle formation using amphiphilic diblock copolymer. OJPolym. Chem. 2022, 12, 43-54. https://doi.org/10.4236/ojpchem.2022.122003
2. E. Yoshida, Polymer nanoarchitectonics for synthetic vesicles with human erythrocyte-like morphology transformation. Colloid Polym. Sci. 2022, 300, 497-508. https://doi.org/10.1007/s00396-022-04958-2
3. E. Yoshida, Perforated giant vesicles composed of amphiphilic diblock copolymer: New artificial biomembrane model of nuclear envelope. Soft Matter 2019, 15, 9849-9857. https://doi.org/10.1039/C9SM01832H
4. E. Yoshida, Fabrication of anastomosed tubular networks developed out of fenestrated sheets through thermo responsiveness of polymer giant vesicles. ChemXpress 2017, 10(1):118, 1-11. https://www.tsijournals.com/articles/fabrication-of-anastomosed-tubular-networks-developed-out-of-fenestrated-sheets-through-thermo-responsiveness-of-polymer-giant-ves.html
5. E. Yoshida, Fabrication of microvillus-like structure by photopolymerization-induced self-assembly of an amphiphilic random block copolymer. Colloid Polym. Sci. 2015, 293, 1841-1845. https://doi.org/10.1007/s00396-015-3600-1
6. E. Yoshida, Morphological changes in polymer giant vesicles by intercalation of a segment copolymer as a sterol model in plasma membrane. Colloid Polym. Sci. 2015, 293, 1835-1840. https://doi.org/10.1007/s00396-015-3577-9
7. E. Yoshida, PH response behavior of giant vesicles comprised of amphiphilic poly(methacrylic acid)-block-poly(methyl methacrylate-random-methacrylic acid). Colloid Polym. Sci. 2015, 293, 649-653. https://doi.org/10.1007/s00396-014-3482-7
8. E. Yoshida, Morphology control of giant vesicles by composition of mixed amphiphilic random block copolymers of poly(methacrylic acid)-block-poly(methyl methacrylate-random- methacrylic acid). Colloid Polym. Sci. 2015, 293, 249-256. https://doi.org/10.1007/s00396-014-3403-9
9. E. Yoshida, Hydrophobic energy estimation for giant vesicle formation by amphiphilic poly(methacrylic acid)-block-poly(alkyl methacrylate-random-methacrylic acid) random block copolymers. Colloid Polym. Sci. 2014, 292, 2555-2561. https://doi.org/10.1007/s00396-014-3297-6
Keywords
Title of class
Advanced Polymer Engineering / Advanced Biotechnology 2
Others (Awards, Committees, Board members)
1. International Best Researcher Award (The American Chamber of Research), 2024年10月20日.
2. Asia's Outstanding Researcher Award (2023) for her study on CO2 capture-induced polymer complexes.
3. The Coating Engineering Editor-in-Chief Award (2011) for her article titled 'Synthesis of Functional Nanoparticles Through Polymer Self-Assembly in Supercritical Carbon Dioxide.
4. The Society of Japanese Women Scientists Award (2007) for creating novel 'non-amphiphilic' polymer micelles by controlled/living radical polymerization.
5. The SPSJ Award for Outstanding Paper in the Polymer Journal (1999) for her article titled 'Synthesis of Poly(ethylene adipate) with a Stable Nitroxyl Radical at Both Chain Ends and Its Application to Living Radical Polymerization.'
6. The Award for Encouragement of Research in Polymer Science (1998) for her study on the macromolecular design based on living radical polymerization using stable nitroxyl radicals.
7. The Award for Promoting Lecture in the Chemical Society of Japan (1997) for her presentation titled 'Synthesis and Characterization of Polytetrahydrofuran-block-Polystyrene Block Copolymers Using Living Radical Polymerization.