Tero, Ryugo
| Affiliation | Department of Applied chemistry and Life Science |
|---|---|
| Title | Professor |
| Fields of Research | Surface physical chemistry |
| Degree | PhD, Graduate School of Science, The University of Tokyo |
| Academic Societies | American Chemical Society, Chemical Society of Japan, Japan Society of Applied Physics, Surface Science Society of Japan, Biophysical Society of Japan |
| tero@ Please append "tut.jp" to the end of the address above. |
|
| Laboratory website URL | https://chem.tut.ac.jp/interface/ |
| Researcher information URL(researchmap) | Researcher information |
Research
Structure and dynamics of artificial cell membrane systems
Lipid bilayers are fundamental structures of cell membranes, and provide reaction fields to membrane proteins relating to the transportation of signal, materials and energy into and/or out of cells. Artificial lipid bilayers on solid materials, called supported lipid bilayers (SLBs), are interfacial system between biomembranes and solid devices. My research target is structures and dynamics of lipids and proteins in lipid bilayer membranes (e.g. lipid diffusion, domain formation/dissipation, peptides and protein assemblies), and they are investigated on the scale of single molecule to micrometer assembly with fluorescence-microscope-based techniques and atomic force microscopy.
Theme1:Effects of substrate properties on supported lipid bilayer (SLB) formation and phase separation
Overview
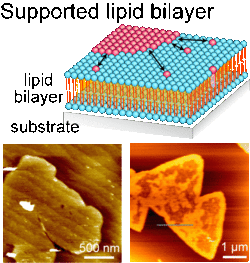 Schematic of supported lipid bilayer (SLB), and AFM topographs of SLBs with two dimensional domains.
Schematic of supported lipid bilayer (SLB), and AFM topographs of SLBs with two dimensional domains.SLBs are formed by vesicle fusion method in my lab. Substrates soaked in a suspension of lipid vesicles (=liposomes) are spontaneously coated by SLB through the vesicle transformation processes from sphere to planar membrane e.g. adsorption, fusion, rupture and spreading. In these processes, interaction between vesicles and substrates are critical factor thus SLB formation highly depends on the substrate conditions, vesicle size and lipid component. Understanding how the substrate physical and chemical properties (structures, roughness, charges, chemical termination, etc) affects to the SLB formation process, and furthermore the two dimensional structures in SLB, is essential for fabricating functional model biomembrane systems.
Selected publications and works
M. W. S. Goh and R. Tero*, "Non-raft submicron domain formation in cholesterol-containing lipid bilayers induced by polyunsaturated phosphatidylethanolamine", Colloids Surf. B, 210, 112235 (11 pages) (2022), 10.1016/j.colsurfb.2021.112235.
M. W. S. Goh and R. Tero*, "Cholesterol-induced microdomain formation in lipid bilayer membranes consisting of completely miscible lipids", Biochim. Biophys. Acta Biomembr., 1863, 183626 (2021), 10.1016/j.bbamem.2021.183626.
Y. Kakimoto and R. Tero*, "Formation of supported lipid bilayers of Escherichia coli extracted lipids and their surface morphologies", Jpn. J. Appl. Phys., 58, SIIB19 (7 pages) (2019), 10.7567/1347-4065/ab1b5f.
R. Tero*, K. Fukumoto, T. Motegi, M. Yoshida, M. Niwano and A. Hirano-Iwata, "Formation of Cell Membrane Component Domains in Artificial Lipid Bilayer", Sci. Rep., 7, 17905 (10 pages) (2017), 10.1038/s41598-017-18242-9.
R. Tero*, R. Yamashita, H. Hashizume, Y. Suda, H. Takikawa, M. Hori and M. Ito*, "Nanopore formation process in artificial cell membrane induced by plasma-generated reactive oxygen species", Arch. Biochem. Biophys., 605, 26-33 (2016), 10.1016/j.abb.2016.05.014.
Keywords
Theme2:In situ observation of molecular diffusion in SLB
Overview
Lateral diffusion of lipid molecules in cell membranes is fundamental process of domain formation and membrane reactions. I use single molecule tracking (SMT) method to observe the lipid diffusion in SLBs in situ. In my experimental setup, SMT is achieved independent of the substrate transparency or refractive index, thus silicon, TiO2 and other oxide materials are available. SMT on nano-structured oxide surfaces are currently underway.
Selected publications and works
Y. Okamoto, S. Iwasa and R. Tero*, "Quenching Efficiency of Quantum Dots Conjugated to Lipid Bilayers on Graphene Oxide Evaluated by Fluorescence Single Particle Tracking",
Appl. Sci., 12, 3733 (2022), 10.3390/app12083733.
T. Motegi*, K. Takiguchi, Y. Tanaka-Takiguchi, T. Itoh and R. Tero*, "Physical Properties and Reactivity of Microdomains in Phosphatidylinositol-Containing Supported Lipid Bilayer", Membranes, 11, 339 (12 pages) (2021), 10.3390/membranes11050339.
T. Motegi*, K. Yamazaki, T. Ogino and R. Tero*, "Substrate-Induced Structure and Molecular Dynamics in a Lipid Bilayer Membrane", Langmuir, 33, 14748-14755 (2017), 10.1021/acs.langmuir.7b03212.
Y. Okamoto, T. Motegi, K. Morita, T. Takagi, H. Amii, T. Kanamori, M. Sonoyama* and R. Tero*, "Lateral Diffusion and Molecular Interaction in a Bilayer Membrane Consisting of Partially Fluorinated Phospholipids", Langmuir, 32, 10712-10718 (2016), 10.1021/acs.langmuir.6b02874.
Keywords
Theme3:Artificial lipid bilayer platform on graphene
Overview
Graphene is a single atomic sheet consisting of sp2 carbon. Its high electron mobility has been attracted researcher's interests, but recently various unique properties of graphene is becoming known. One of them is the unordinary fluorescence quenching function. Emission of fluorescence probes are quenched independent of the wave length, and the efficient distance is much further than the conventional FRET between molecules. We are developing lipid bilayer platform on graphene and graphene oxide to develop a new method for lipids and proteins in and on lipid bilayer membranes.
Keywords
Title of class
Fundamental Inorganic Chemistry 1, 2 (B1); Fundamental Physical Chemistry 3, 4 (B2); Physical Chemistry 4 (B3); Advanced Microscopic Imaging Technology (M)・Advanced Molecular Function Chemistry 2 (D)