
ここからコンテンツです。

Development of rapid and simultaneous diagnosis of COVID-19/influenza diseases by manipulating microfluidic flow with a microfluidic chip
Daigo Natsuhara
A research team consisting of Daigo Natsuhara, a second year Ph.D. student and Professor Takayuki Shibata etc. at the Department of Mechanical Engineering, Toyohashi University of Technology, and Professor Hirotaka Kanuka, etc. of the Jikei University School of Medicine has developed a microfluidic chip which is capable of simultaneous diagnosis of COVID-19 and influenza diseases, by applying the microfluidic chip technologies. The team has built a theoretical model that manipulates microfluidic flow with an extremely simple microchannel design and has established an optimal design theory for microfluidic chips. Further, using the diagnostic device they developed, they performed genetic amplification experiments on four types of infectious diseases, including COVID-19, and demonstrated that multiplexed rapid and simultaneous diagnosis was possible within 30 minutes. The device is not limited to human infectious diseases, but can be utilized for genetic diagnoses in a range of fields (e.g. agriculture, farming, and fisheries industries, food industry, and health and medical care).
As shown during the global COVID-19 pandemic, viral diseases cause immense public concern and have a massive impact on economic activity. In this research, with the goal of creating technology to make our lives safer and more secure, the team developed a diagnostic technology that enables anyone to detect viral diseases in a rapid, simple, and low-cost way. What is more, it can be carried out anywhere and at any time.
The LAMP (Loop-Mediated Isothermal Amplification) method*1 is a genetic test technology. This simple test method, unlike the commonly used PCR test, does not require expensive equipment, such as for accurate temperature control. Moreover, the test can be conducted on site because it allows genetic amplification at a constant temperature for a constant length of time (60 to 65℃, 30 minutes to an hour or so). However, to diagnose multiple types of viruses, the conventional LAMP method entails considerable effort to perform as many preparations of samples and reagents and genetic amplification reactions as the number of analytes, requiring expert knowledge and skills.
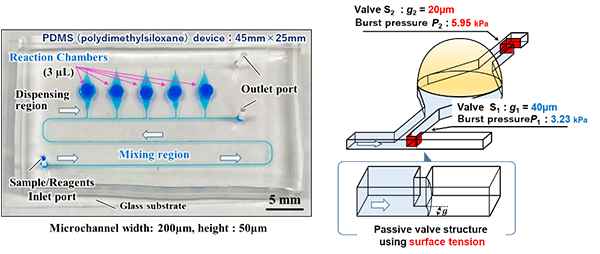
Therefore, the research team of Toyohashi University of Technology and the Jikei University School of Medicine developed a polydimethylsiloxane (PDMS)-based multiplexed genetic diagnostic device (size: 45mm x 25mm, reaction chamber: 3µL x 5 pieces) (refer to the photo in the figure) by applying microfluidic chip technology. It autonomously, equally, and accurately dispenses samples and reagent into an array of reaction chambers simply by introducing the liquid, a mixture of an extremely small amount of sample extracted from the analytes and a reagent, into the diagnostic device. By heating the device in warm water (at 60 to 65℃, for 30 minutes to an hour or so), it is capable of achieving simultaneous diagnoses of multiple types of viruses with only one operation (one work process per sample).
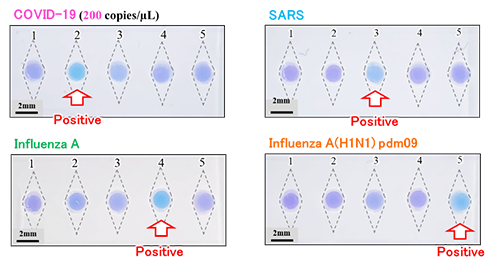
The genetic diagnosis result shown in the figure indicates that four types of infectious diseases including the COVID-19 (seasonal influenza A, SARS, and influenza H1N1 pdm09) have been successfully detected with this diagnosis device. Only the reaction chamber that reacted when a sample containing the gene of each virus was introduced turned sky blue (denoting a positive reaction) after 30 minutes, which means that visual detection is possible.
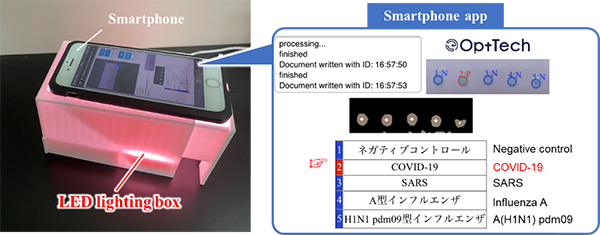
In addition, to support on-site diagnoses, the team has developed a smartphone app, which automatically diagnoses the reaction as positive or negative based on photographs taken with a smartphone camera, jointly with OptTech LLC. (Toyohashi, Aichi; founded by a graduate from the university). As shown in the figure, the diagnosis device is capable of producing an easy automatic test result diagnosis (positive or negative), by placing the device after LAMP reaction in a simple LED illumination device and taking photographs with a smartphone. As a result, it is expected that anyone will be able to easily perform the test, anywhere and at any time.
Note: The LAMP method is a loop-mediated isothermal amplification method developed by Eiken Chemical Co., Ltd. (https://www.eiken.co.jp/). Four to six types of primers are set for six to eight areas of a targeted gene to amplify the gene at a constant temperature (60 to 65℃) through strand displacement reaction.
In the future, aiming to commercialize the diagnosis device, the research team will develop devices capable of multiplexed rapid diagnosis of variants of the COVID-19 and human infectious diseases for a safe life during and after the COVID-19 pandemic. To provide technologies that contribute to food safety and security, it will aim to realize multiple simultaneous diagnoses of food allergic substances (seven specific raw materials: wheat, soba, peanut, egg, milk, shrimp, and crab) and food poisonous fungi (e.g. salmonella and O-157).
This research was partially supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP20he0622021 (Development of µTAS-based device for rapid detection of SARS-CoV-2; representative: Professor Hirotaka Kanuka). We express our cordial gratitude for the support.
Reference
Daigo Natsuhara, Ryogo Saito, Hiroka Aonuma, Tatsuya Sakurai, Shunya Okamoto, Moeto nagai, Hirotaka Kanuka, and Takayuki Shibata, (2021) A method of sequential liquid dispensing for the multiplexed genetic diagnosis of viral infections in a microfluidic device. Lab on a Chip, 21(24),
4779-4790, 10.1039/d1lc00829c
マイクロ流路チップで微小流体を自在に操り、新型コロナ・インフルエンザ同時迅速診断を実現
夏原 大悟
豊橋技術科学大学機械工学専攻 博士後期課程1年の夏原大悟、機械工学系 柴田隆行教授らと東京慈恵会医科大学 嘉糠洋陸教授らの研究チームは、マイクロ流体チップテクノロジーを応用し、新型コロナウイルスとインフルエンザウイルスを同時に診断できるマイクロ流路チップを開発しました。マイクロスケールの微小な流体を極めて単純な流路形状で制御する理論モデルを構築し、マイクロ流路チップの最適設計手法を確立しました。さらに、開発した診断デバイスを用いて、新型コロナウイルスを含む4種類の感染症ウイルスの遺伝子増幅実験を行い、30分以内での多項目同時迅速診断が可能なことを実証しました。本デバイスは、ヒト感染症に限らず、様々な分野(農業・畜産・水産業、食品産業、健康・医療など)での遺伝子診断に活用可能です。
新型コロナウイルス感染症(COVID-19)の世界的な大流行(パンデミック)が引き起こしたように、ウイルス感染症がもたらす人々への恐怖や、経済活動へ与える影響は計り知れません。本研究では、安全・安心な暮らしを守るための支援技術として、迅速・簡便・低コストに誰でも、いつでも、どこででも手軽に感染症検査ができる診断技術を開発しました。
遺伝子検査技術の一つとして、LAMP(Loop-Mediated Isothermal Amplification)法注)があります。本手法は、一定の温度・所要時間(60~65℃、30分~1時間程度)で遺伝子増幅が行えることから、広く普及しているPCR検査のように高価な精密温度制御装置などが不要で、オンサイトでも実施ができる簡易な検定法です。しかし、従来のLAMP法では、複数項目のウイルス診断を行うためには、検査対象の数だけ検体・試薬の調整と遺伝子増幅反応を行う煩雑さがあり、その作業には専門的な知識やスキルが必要です。
そこで、豊橋技術科学大学と東京慈恵会医科大学の研究チームでは、マイクロ流体チップテクノロジーを応用し、シリコーン樹脂(PDMS)製のマルチプレックス遺伝子診断デバイス(サイズ45mm×25mm、反応容器3µL×5個)を開発しました(図の外観写真参照)。検査対象から抽出した極微量の検体と試薬の混合液を診断デバイスに導入するだけで、自律的に複数の反応容器内に検体・試薬を均等かつ高精度に分注し、湯中にて加温(60~65℃、30分~1時間程度)することで、1回の操作(1検体1作業工程)で複数種類のウイルスの同時診断を可能としました。
図に示す遺伝子診断結果では、新型コロナウイルスを含む4種類の感染症ウイルス(A型インフルエンザ、SARS、H1N1 pdm09型インフルエンザ)を本診断デバイスで検出することに成功しています。各ウイルス遺伝子を含むサンプルを導入したときに対応する反応容器のみが30分で陽性を示す水色に変化しており、目視での診断が可能となっています。
さらに、オンサイトでの診断に対応するために、スマートフォンの撮影画像から陽性・陰性を自動で判定するためのスマホアプリをOptTech社(愛知県豊橋市:本学卒業生が起業)と共同開発しました。図のように、遺伝子増幅後の診断デバイスを簡易LED照明装置内に設置し、スマホで写真を撮影するだけで、陽性か陰性かの検査結果を自動診断できるようになりました。これによって、誰でも、いつでも、どこででも手軽に検査ができるようになることが期待できます。
注)LAMP法は、栄研化学株式会社(https://www.eiken.co.jp/)が開発した等温遺伝子増幅法です。標的となる遺伝子の6~8つの領域に対して4~6種類のプライマーを設定し、鎖置換反応を利用して一定温度(60~65℃)で遺伝子を増幅します。
今後は、本診断デバイスの実用化を目指して、ウィズコロナ・アフターコロナ時代の安心な暮らしのために、新型コロナウイルスの変異株やヒト感染症ウイルスの多項目迅速診断が行えるデバイスの開発を行います。さらに、食の安全・安心に資する技術の提供を目的として、食物アレルギー物質(特定原材料7品目:小麦、そば、落花生、卵、牛乳、えび、かに)や食中毒菌(サルモネラ菌、O-157など)の多項目同時診断を目指します。
本研究は、AMEDの課題番号JP20he0622021(検査ギャップ解消を指向した新型コロナウイルス検出用マイクロ流路チップの開発/代表:嘉糠 洋陸 教授)の支援を受けて行った研究です。ここに深く謝意を表します。
Researcher Profile

| Name | Daigo Natsuhara |
|---|---|
| Affiliation | Department of Mechanical Engineering |
| Title | Doctor course student |
| Fields of Research | Microfluidics / µTAS |
ここでコンテンツ終わりです。
