
ここからコンテンツです。

Progress Towards the Realisation of Advanced Higher Capacity Li-ion Batteries
Binder-less tin phosphide/carbon composite film electrodes by aerosol deposition processBy Ryoji Inada
Associate Professor Ryoji Inada and his research team at the Toyohashi University of Technology have successfully fabricated a binder-less tin phosphide (Sn4P3)/carbon (C) composite film electrode for lithium-ion batteries via aerosol deposition. The Sn4P3/C particles were directly solidified on a metal substrate via impact consolidation, without applying a binder. Charging and discharging cycling stabilities were improved by both complexed carbon and a controlled electrical potential window for lithium extraction. This new material could help realize advanced lithium-ion batteries of higher capacity.
Lithium-ion (Li-ion) batteries are widely used as a power source in portable electronic devices. They have recently attracted considerable attention because of their potential to be employed in large-scale uses, such as acting as a power source for electric vehicles and plugin hybrid electric vehicles, and as a stationary energy storage system for renewable energy. To realize advanced Li-ion batteries with higher energy density, anode materials with higher capacity are required. Although a few Li alloys such as Li–Si and Li–Sn, whose theoretical capacity is much higher than that of graphite (theoretical gravimetric capacity = 372 mAh/g), have been extensively studied, they generally result in poor cycling stability due to the large variation in volume during charging and discharging reactions.
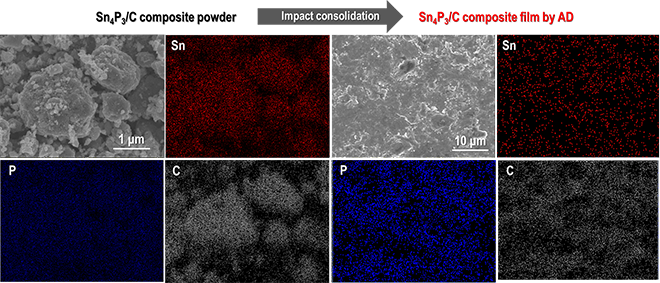
Tin phosphide (Sn4P3) (theoretical gravimetric capacity = 1255 mAh/g) with layered structure, generally used as a high-capacity alloy-based anode material for Li-ion batteries, has an averaged operational potential of ~0.5 V vs. Li/Li+. Reports indicate that complexing carbon materials with nano-structured Sn4P3 particles significantly enhance the cycling stability. Generally, electrodes used in batteries are fabricated by coating a slurry consisting of electrode active materials, conductive carbon additives, and binders, on metallic foils. For carbon complexed Sn4P3 (Sn4P3/C) anodes (such as those reported in the literature), the weight fraction of the active materials in an electrode is decreased by approximately 60~70 % because of the use of significant quantities of conductive additives and binders to achieve stable cycling. Consequently, the gravimetric specific capacity per electrode weight (including those of conductive carbon additives and binders) is decreased significantly.
Associate Professor Ryoji Inada and his research team at the Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, have successfully fabricated a binder-less Sn4P3/C composite film electrode for Li-ion battery anodes via aerosol deposition (AD). In this process, the Sn4P3 particles are complexed with acetylene black using a simple ball-milling method; the obtained Sn4P3/C particles are then directly solidified on a metal substrate via impact consolidation without adding any other conductive additives or binders.
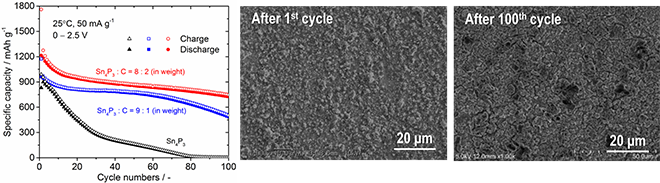
This method enables enhancement of the fraction of Sn4P3 in the composite to above 80%. Furthermore, structural change of the composite electrode is reduced and cycling stability is improved by both complexed carbon and a controlled electrical potential window for lithium extraction reaction. The Sn4P3/C composite film fabricated by the AD process maintains gravimetric capacities of approximately 730 mAh g-1, 500 mAh g-1, and 400 mAh g-1 at 100th, 200th, and 400th cycles, respectively.
The first author Toki Moritaka is quoted as saying, "Although optimizing the deposition conditions was difficult, useful information on enhancement of cycling stability of the Sn4P3/C composite film electrode fabricated by the AD process was obtained. The complexed carbon functions not only as a buffer to suppress the collapse of electrodes caused by the large variation in the volume of Sn4P3, but also as an electronic conduction path among the atomized active material particles in the composite."
"This process is an effective means to increase the capacity value per electrode weight. We believe there is scope for improvement of the electrochemical performance by the size and content of the carbon in Sn4P3/C used in composite film fabrication by the AD process. We are now trying to optimize the complexed carbon content and increase the composite film thickness," quotes Associate Professor Ryoji Inada.
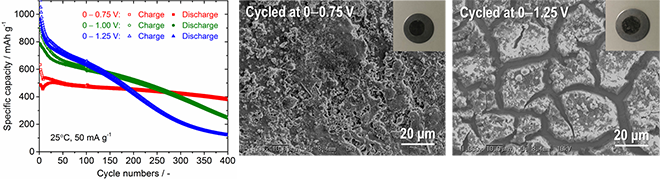
The findings of this study may contribute to the realization of advanced Li-ion batteries of higher capacity. Moreover, because not only Li but Na can also be stored in and extracted from Sn4P3 by similar alloying and dealloying reactions, the Sn4P3 electrode can be employed in next-generation Na-ion batteries at much lower costs.
This work was partly supported by Grant-in-Aids for Scientific Research (Grant No. 16K06218 and 16KK0127) from the Japan Society for the Promotion of Science (JSPS).
Reference
Toki Moritaka, Yuh Yamashita, Tomohiro Tojo. Ryoji Inada, Yoji Sakurai (2019). Characterization of Sn4P3–Carbon Composite Films for Lithium-Ion Battery Anode Fabricated by Aerosol Deposition: Nanomaterials, 9(7), 1032.10.3390/nano9071032.
http://dx.doi.org/10.3390/nano9071032
次世代型高容量リチウムイオン電池の実現に向けて
リン化錫・炭素複合膜電極のバインダレス形成による長寿命化By 稲田 亮史
豊橋技術科学大学の研究グループは、エアロゾル・デポジション(AD)法を用いてバインダ(結着材)を用いず、リン化錫(Sn4P3)/ カーボン(C)複合膜を金属基板上に形成することに成功しました。原料であるSn4P3/C粒子が基板に衝突した際の衝撃により変形・固化することにより、複合膜が形成できたと考えられます。カーボン複合化とリチウム脱離反応時の電圧の制御を組み合わせることで、充放電サイクル特性が向上することを確認しました。本成果は、次世代型高容量リチウムイオン電池の実現に貢献すると考えられます。
リチウムイオン電池は、携帯用小型電子機器の電源として広く使用されており、最近では電気自動車、プラグインハイブリッド電気自動車、および定置型蓄電システム等の中・大型電源としての用途展開が加速しています。大容量な次世代型リチウムイオン電池の実現には、容量の高い負極材料の開発が望まれます。シリコンや錫は、リチウムとの合金化反応を通じて、現行の黒鉛負極(理論容量:372mAh/g)よりもはるかに大きな理論容量を示すため精力的に研究されています。しかしながら、合金化(充電)・脱合金化(放電)時の体積変化が大きく、サイクル安定性が低いことが課題となっています。
リン化錫Sn4P3(理論容量:1255mAh/g)は、リチウム基準で約0.5 Vの電位で作動する高容量合金系負極材料の一つです。ナノサイズ化したSn4P3をカーボン(C)と複合化することで、そのサイクル安定性が向上することが既に確認されています。一般的にリチウムイオン電池の電極は、充放電を担う材料(活物質)と導電助剤、バインダ(結着材)および溶剤を混合してスラリーを作製し、これを金属箔上に塗布・乾燥する工程で作製されます。Sn4P3/C複合粒子を活物質とした先行研究では、サイクル安定性向上のため多量の導電助剤・バインダを混合しており、最終的な電極内での活物質の充填率(重量)は60~70%となり、電極総重量あたりの容量値が低下するという課題があります。
豊橋技術科学大学電気・電子情報工学系の稲田亮史准教授と森高冬毅(2019年3月博士前期課程修了)の研究グループは、エアロゾル・デポジション(AD)法を用いてバインダ(結着材)を用いず、Sn4P3/C複合膜を金属基板上に形成することに成功しました。Sn4P3粒子にカーボン材料(アセチレンブラック)を簡便なボールミル処理によって複合化し、衝撃固化を介して導電助剤やバインダを加えることなく、金属基板上に固化させました。本手法により、電極内での活物質の充填率は80%以上に調整しました。カーボンの複合化および放電(脱合金化)電位の制御により、充放電サイクル時の電極構造の変化が抑制され、充放電サイクル安定性の顕著な向上を確認しました。結果として、100サイクル後で黒鉛負極の2倍に相当する730mAh/g、200サイクル後で500mAh/g、400サイクル後で400mAh/gの可逆容量を保持することができました。
筆頭著者の森高冬毅(2019年3月本学博士前期課程修了)は、「成膜条件の検討に苦労しましたが、Sn4P3/C複合膜電極のサイクル安定性向上に向けた有益な知見が得られました。複合化したカーボンは、Sn4P3の大きな体積変化によって誘発される電極崩壊を抑制すると共に、電極内で変形・微粒化した活物質粒子間の電気伝導パスを担っていると考えます」と説明しています。
また、指導教員である稲田亮史准教授は、「電極総量あたりの容量値を高める上で本プロセスは有効な手段であり、複合膜の作製に使用するSn4P3/C複合粒子のサイズやカーボンの種類・量の調整によって更なる性能改善が達成可能と考えています。電極の厚膜化と併せて、更なる性能改善に向けた検討を進めています」と、述べています。
本研究の成果は、次世代型高容量リチウムイオン電池の実現に貢献するものと考えます。また、Sn4P3はリチウムのみならず資源的制約の少ないナトリウムについても合金化・脱合金化反応を示すため、リチウムイオン電池よりも低コスト化が可能なナトリウムイオン電池用電極への応用も期待できます。
本研究の一部は日本学術振興会(JSPS)科学研究費助成事業(課題番号16K06218および16KK0127)の支援の下で実施されたものです。
Researcher Profile

| Name | Ryoji Inada |
|---|---|
| Affiliation | Department of Electrical and Electronic Information Engineering |
| Title | Associate Professor |
| Fields of Research | Energy Conversion Engineering / Electirical and Electronic Material Engineering / Electrochemistry |
ここでコンテンツ終わりです。
