
ここからコンテンツです。

Wrap an Electrode Material for Li-ion Battery into the Inner Spacing of Carbon Nanotube
Electrochemical characterization of a high capacitive electrode for lithium ion batteries using phosphorus-encapsulated carbon nanotubes By Tomohiro Tojo
Tomohiro Tojo, Assistant Professor, and his group at the Toyohashi University of Technology have designed a unique lithium ion battery (LIB) electrode, where red phosphorus is packed into carbon nanotubes (CNTs). the new electrodes displayed reversible electrochemical reactions and maintained a relatively high structural stability of red phosphorus in the nanotubes even after the fiftieth charge–discharge cycle. The charge-discharge capacities are at least twice as high as for graphite in commercial LIBs. Therefore, it can be said that this new electrode material for LIBs with high capacity shows promise.
In this experiment, the electrochemical performance of a new type of lithium ion batteries (LIBs) was demonstrated. These batteries are constructed using phosphorus-encapsulated carbon nanotube electrodes, whereby ultra high capacity red phosphorus is introduced into the inner spacing of carbon nanotubes (CNTs) with a tubular structure. The new electrodes indicated an improvement in the electrochemical reactivity of red phosphorus when accessible pathways of lithium ions, i.e., nanopores, were formed onto the sidewalls of the CNTs where the red phosphorus was encapsulated. Furthermore, the charge–discharge profiles and structural analysis revealed reversible electrochemical reactions as well as a relatively high structural stability of red phosphorus in the nanotubes even after the fiftieth charge–discharge cycle. The charge–discharge capacity values achieved are comfortably double those achieved by graphite used in commercial LIBs. Therefore, this new electrode material appears promising for use in high capacity LIBs.
Red phosphorus has attracted attention as a higher capacitive electrode material for LIBs because it can deliver a theoretical capacity approximately seven times higher than that of the graphite currently used as a commercial electrode material for LIBs. The large difference in the capacity is thought to be due to an acceptable amount of lithium ions in the structures of graphite for LiC6 or phosphorus for Li3P. However, red phosphorus suffers enormous volumetric changes, pulverization, and peeling off during lithium ion insertion and extraction processes, resulting in rapid capacity fading due to the decrement in the amount of electrochemically reactive red phosphorus. Additionally, while electrons move onto the electrode during lithium ion insertion/extraction, red phosphorus has a disadvantage in terms of energy loss because of its low electronic conductivity.
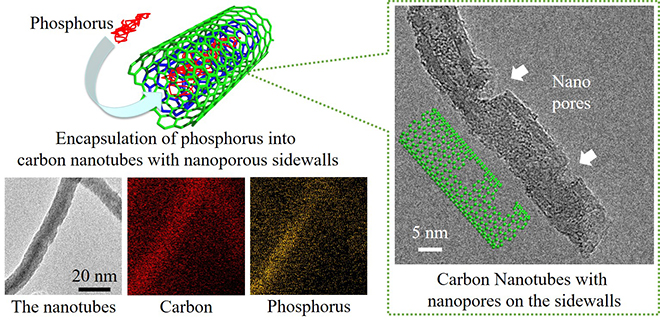
As shown in Fig. 1 (left), Tomohiro Tojo and his colleagues at the Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, have synthesized unique structures in which red phosphorus is encapsulated into the inner spacing of CNTs to prevent its peeling off from the electrode and improve its electronic conductivity. In order to further improve the electrochemical reactivity of red phosphorus through accessible pathways of lithium ions, nanopores (<5 nm) were also formed onto the sidewalls of the phosphorus-encapsulated CNTs as shown in Fig. 1 (right). After phosphorus encapsulation, Fig. 1 (left) shows that the phosphorus atoms were distributed inside the nanotubes, confirming the structural stability of red phosphorus.
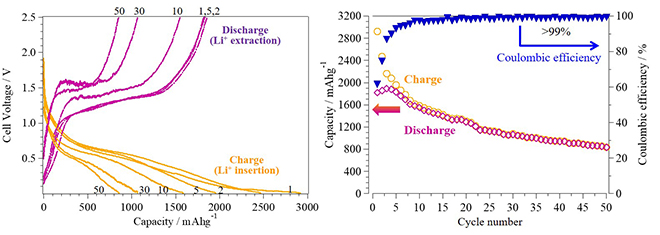
Using phosphorus-encapsulated CNT electrodes, a reversible capacity of approximately 850 mAh/g was achieved at the fiftieth charge–discharge cycle, as depicted in Fig. 2 (left). This was a value at least two times higher than that of graphite electrodes. Fig. 2 (right) shows the estimated ratio of charge and discharge capacities (Coulombic efficiencies) of >99% after the tenth cycle and the subsequent cycles, which indicates a high reversibility of charge–discharge reactions on red phosphorus. However, the charge–discharge capacities gradually decreased with the number of cycles because of the dissociation of some P–P bonds and other side reactions on the surface of phosphorus and the CNTs. Interestingly, the phosphorus-encapsulated CNT with nanopores facilitated a significant improvement in electrochemical performance when compared with the phosphorus-encapsulated CNT without nanopores. This is suggested to be due to the high reactivity of red phosphorus with lithium ions through the nanopores on the sidewalls. After the charge–discharge cycles, red phosphorus was observed to be inside the nanotubes, as is the case shown in Fig. 1 (left).
We have proposed phosphorus-encapsulated CNTs as an electrode material for LIBs with high capacity, even though additional improvements in the structures are required to achieve long-term cycling without capacity fading. Further studies will be performed on the utilization of such electrodes.
This research was supported financially by the Murata Science Foundation, the Public Interest Tatematsu Foundation, and the Public Foundation of the Chubu Science and Technology Center.
Reference
Tomohiro Tojo, Shinpei Yamaguchi, Yuki Furukawa, Kengo Aoyanagi, Kotaro Umezaki, Ryoji Inada, and Yoji Sakurai, Electrochemical Performance of Lithium Ion Battery Anode Using Phosphorus Encapsulated into Nanoporous Carbon Nanotubes. Journal of The Electrochemical Society, 165(7), A1231-1237 (2018).
https://doi.org/10.1149/2.0351807jes
カーボンナノチューブ内にリチウムイオン電池電極材料を詰め込む
リン内包カーボンナノチューブを用いた高容量リチウムイオン電池電極の電気化学特性評価By 東城 友都
豊橋技術科学大学 電気・電子情報工学系 東城友都助教らの研究グループは、円筒状炭素材料であるカーボンナノチューブ(CNT)の中空孔に、高容量リチウムイオン電池(LIB)電極材料である赤リンを詰め込み、充放電試験を行いました。CNTの側壁に、10億分の1メートル(ナノメートル)の小さな孔を開けることで、リチウムイオンの出入りが容易となり、CNT内部の赤リンの反応性が向上することが示されました。50回の充放電試験を繰り返し行なっても、可逆な充放電反応を示し、赤リンはCNT内部に比較的安定に存在することが明らかとなりました。電池容量は、現行のLIB電極材料のグラファイトに比べ2倍以上となり、本研究は、高容量LIB電極材料の提案につながりました。
赤リンをリチウムイオン電池(LIB)電極材料に用いた場合、現行のLIB電極に使用されているグラファイトに比べて約7倍の充放電容量を示す可能性があるため、赤リンは高容量LIB電極材料として注目を集めています。グラファイトの場合、6個の炭素原子が1個のリチウムイオンを吸蔵しますが、赤リンの場合、1個のリン原子が3個のリチウムイオンを吸蔵できます。このリチウムイオン吸蔵量の差から、赤リンを用いたLIBは高容量化を達成できることが考えられます。しかし、赤リンはその分、リチウムイオンの吸蔵・放出による体積変化が大きく、繰り返し充放電反応を生じると赤リン粒子の亀裂・剥離・脱落が起こります。その結果、充放電反応に寄与する赤リン粒子の量が減少してしまい、電池容量の急激な低下が問題となります。また充放電反応時に電極では電子のやり取りも生じますが、赤リンが電気を流しにくい性質(絶縁体)であるため、エネルギーロスが大きいことが問題となります。
そこで東城友都助教らの研究グループは、図1(左)に示すように、赤リン粒子の亀裂や剥離に伴う粒子脱落を抑止するために、カーボンナノチューブ(CNT)の中空孔に赤リンを詰め込んだ構造を持つ材料を合成しました。電気を流しやすいCNTは、赤リン粒子の電気的弱点を補う働きも担います。電池として充放電させる際に、リチウムイオンの移動を円滑にして、赤リンの電気化学反応性を向上させるために、図1(右)のように予めCNTの側壁にナノサイズ(<5nm)の孔を形成しても、図1(左)の分析画像からCNTの中空孔に赤リンが安定に存在することも確認できました。
この材料をLIB電極に適用することで、50回の充放電試験においても、図2(左)の通り、約850 mAh/gの可逆容量が得られ、グラファイトの2倍以上の容量を示しました。また図2(右)に示す通り、10回の充放電試験以降、充放電効率(クーロン効率)は99%以上の高い値を示し、充放電反応の可逆性が高いことがわかりました。しかし、充放電を繰り返し行うと、充放電容量が徐々に低下していきました。この原因として、赤リン粒子が劣化していることや、副反応に電荷が消費されていることが考えられます。ただし、側壁に孔を開けていないCNTに赤リンを埋め込んだ電極よりも、赤リンの電気化学反応性が向上し、格段に充放電性能が向上していることが判明しました。また図1(左)と同様に、充放電後にも赤リン粒子がCNTの中空孔に存在している様子が観察され、赤リンの構造安定化を達成できました。
本研究では高容量LIB電極材料として、CNTの中空孔に赤リンを埋め込んだ構造を提案しましたが、実用時において充放電反応を長期間繰り返す場合には、更なる電極構造の改質が必要です。今後も、このような高容量LIB電極材料の研究を引き続き進めていく予定です。
本研究は公益財団法人 村田学術振興財団、公益財団法人 立松財団、公益財団法人 中部科学技術センターの支援を受けて遂行されました。
Researcher Profile

| Name | Tomohiro Tojo |
|---|---|
| Affiliation | Department of Electrical and Electronic Information Engineering |
| Title | Assistant Professor |
| Fields of Research | Energy Conversion Engineering / Electirical and Electronic Material Engineering |
ここでコンテンツ終わりです。
