
ここからコンテンツです。

Overcoming a major obstacle to the practical application of regenerative medicine: Paving the way for the mass production of iPS Cells
Rika Numano

There are high hopes that the innovative approach of using iPS cells for regenerative medicine will be capable of radically transforming the treatment of diseases. However, there are many barriers to its practical application. One of the major problems is that mass production of iPS cells with homogeneous properties cannot be efficiently achieved. Associate Professor Dr. Rika Numano has developed a water-in-oil droplet electroporation as a novel method of overcoming this obstacle, and is now working to achieve the mass production of iPS cells. When voltage is applied to an aqueous droplet containing cells and Yamanaka factors (reprogramming genes), tiny pores are formed on the surface of the cells. The genes can then be introduced into the cells through these transient pores in the cell membranes. This new technique has several advantages over conventional transfection techniques, in that it can be performed relatively easily using fewer cells, and in that it has a lower chance of inducing cancer. As a result there is growing interest in the potential of this method to realize the development of mass-produced implantable iPS cells in the future.
Interview and report by Madoka Tainaka
Creating iPS cells conveniently without using viruses
Professor Shinya Yamanaka of Kyoto University was awarded the 2012 Nobel Prize in Physiology or Medicine for his famous discovery that mature somatic cells such as skin or blood cells can be reprogrammed to become pluripotent iPS cells by introducing four transcriptional factors. Regenerative medicine is an innovative field of medicine which utilises iPS cells. It can potentially restore or replace damaged or lost physical functions as well as completely curing diseases or healing injuries.
A lot of clinical research has been conducted in the field of iPS cells, but there are some obstacles to its practical use, namely the difficulty in generating iPS cells that display consistent characteristics, and a very low production rate (about 1%).
Conventionally, the generation of iPS cells utilized viruses to introduce Yamanaka factors into cells, but these factors are also capable of inducing cancer in cells and creating tumors. Concerns have also been raised about the risk of parts of the viruses’ gene sequence remaining in cells. Currently, there are various methods being developed for generating iPS cells without using viruses, but all of these methods have demonstrated even poorer rates of production efficiency. In addition, the generation and culturing of iPS cells can only be done by trained experts, and these activities require specialized conditions in facilities in order to prevent contamination.
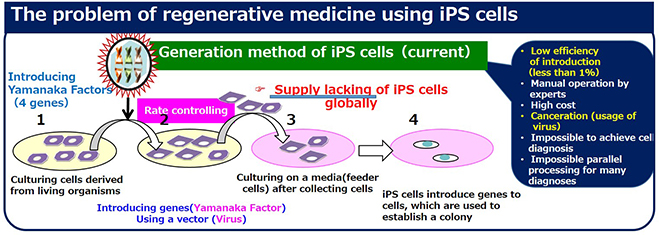
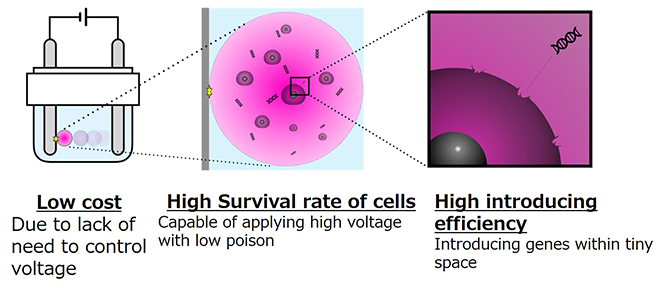
Dr. Numano explains, "We have developed a novel method of generating iPS cells by using water-in-oil droplet electroporation. Cell membranes are made of layers of oils. We have found that by momentarily applying several kilovolts to cells, with about the same voltage as static electricity, the cell membrane loosens and forms transient pores through which the Yamanaka factors can pass. This technique does not require the use of viruses.
However, commercially available electroporation equipment requires a costly pulse generator and sends out high-voltage pulses that kill more than half of the cells. Therefore, rather than implement a method using commercial electroporation equipment, we decided to develop a machine that uses direct-current electric fields to generate iPS cells in a more cell-friendly, convenient and efficient way."
Aiming for chip-based mass production of iPS cells
The procedure for this novel technique is to take an aqueous droplet several microliters in volume containing cells and four Yamanaka factors, put this droplet inside insulating oil, and then apply a direct current from a metal electrode. When the aqueous droplet is exposed to an electric field between a pair of electrodes, the droplet moves back and forth in a rapid bouncing motion between the positive and negative electrodes several hundred times in a minute.
“This technique uses the basic physical property that oil does not mix with water. When the electric field is first applied, the droplet might move to the negative electrode, for example. However, the machine switches the polarity after the droplet contacts with this electrode, and so the droplet is repelled by the negative electrode and moves toward the positive electrode. This cycle is repeated many times, making the droplet move in a bouncing motion. This creates a minute electric current that loosens the cell membranes, allowing for the Yamanaka factors to be introduced into the cells.
Although this reactor is extremely small in size — a water droplet just a few microliters in volume — the droplet contains about ten thousand cells as well as the four types of genes known as Yamanaka factors. This arrangement allows for these genes to be efficiently introduced into the cells. The droplet is insulated by the oil surrounding it, and so there is no risk of contamination in the reactor,” explains Dr. Numano.
One of the most striking points of this new method is that despite a high voltage being used to move the droplet, the electric current in the droplet is very small, and has little influence on the cells.
"We think that if we can conduct the same process in an even smaller reactor, such as picoliter-scale droplets in the micro-channels of a chip, we could further improve the diffusion efficiency of genes, allowing for better introduction of the genes into the cells.
Micro-channels are a completely closed system, with no danger of contamination. They also allow for Yamanaka factors to be introduced into the same cells, enabling a large number of iPS cells to be produced in a short period of time," she says.
This water-in-oil droplet electroporation was developed in a joint research project with Professor Takayuki Shibata and Assistant Professor Hirofumi Kurita from Toyohashi University of Technology. Professor Takayuki Shibata is a bio-MEMS (Micro Electro Mechanical System) researcher working on microneedle arrays for cells, and Assistant Professor Hirofumi Kurita is working on applications of electrostatic forces in life science. A patent has already been obtained for the device, and work is underway together with the electroporator manufacturer Nepa Gene Co., Ltd., in order to realize a practical implementation.
Dr. Numano adds, "Researchers at Juntendo University are conducting clinical research in regenerative medicine using iPS cells to treat Parkinson's Disease. We have requested Professor Wado Akamatsu, from the Center for Genomic and Regenerative Medicine in Juntendo University, to investigate the characteristics of functional differentiation in the iPS cells that we have generated.
Apart from Juntendo University, many clinical studies are currently being conducted at Kyoto University, Osaka University, Keio University, and other institutions in order to seek treatments for neurodegenerative diseases that are considered difficult to cure. We also hope to accelerate our research on mass production methods for iPS cells in order to make the fruits of our research publicly available as soon as possible."
Utilizing this technology to treat diseases by manipulating many cells at once
Before she switched to working with iPS cells, the main focus of Dr. Numano's research was on circadian rhythms in mammals.
"All animals on the earth have a circadian rhythm, which is an internal clock that works in 24-hour cycles. The circadian rhythm is regulated by some 20 types of genes known as clock genes. These clock genes work together within the brains of mammals over 24-hour cycles. This is a very robust system, and is not easily disturbed by minor genetic mutations. If we want to control clock genes, we need to manipulate multiple genes at the same time," Dr. Numano says.
For example, when flying from Japan to the US, our eyes are stimulated by light from the outside, which influences the clock genes in our brain in order to make adjustments to follow local time. However, it is difficult to shift the entire human biological clock by many hours in an instant. As a result, we become jet-lagged because it takes longer for the tissues outside of the nervous system to adjust to the local time.
"I have been working on analyzing the mechanism of the circadian rhythm. I have thought a lot about finding ways to adjust the circadian rhythm instantly. This would allow us to control physiological processes, such as to cure jet lag instantly, or to discover treatments for diseases that are related to the circadian rhythm."
In her research, she came across the electroporation method, which can manipulate many genes at once. This method may allow genes to be introduced into CAR (Chimeric antigen receptor) T cells—a type of white blood cell that specifically attacks cancer cells—to improve their abilities, meaning that this method could also be employed for cancer treatment.
"In addition to iPS cells, this electroporation method can be applied to many other types of genetic manipulation. I would like to conduct further research to refine several aspects of the method, such as identifying how many more genes we can introduce in total, and the types of cells that can be regulated with the method," said Dr. Numano about the future prospects of her research.
Reporter's Note
Dr. Numano grew up in a family with many physicians: her father was a cardiologist and her mother was a pediatrician. She recalls her father’s disappointment when he came home from his university hospital if one of his patients had died that day.
She says, "I thought of becoming a physician as well. However, through witnessing my parents’ experiences, I decided to go into basic research where I could work on ways to fundamentally treat or prevent diseases."
The Human Genome Project commenced when she was a student, and many other research efforts made progress in treating disease preventatively or at early stages. She obtained her doctorate degree while working in the laboratory of our previous Dean Yoshiyuki Sakaki, a prominent Japanese researcher who took a leading role in the Human Genome Project. After graduating, she came to Toyohashi University of Technology, and she has worked over a decade in collaboration with many researchers and companies in order to achieve her dream. Her bright, graceful smile offers the reassurance of an expert physician. We look forward to witnessing further successes in her career.
再生医療普及のカギを握る「iPS細胞の量産化」への挑戦
iPS細胞を用いた再生医療は、病気を根本から治療する革新的な医療として大きな期待が寄せられている。しかし、その実用化には多くの壁が存在する。その一つが、均質な性質を持つ iPS細胞を大量に効率よく量産することができないことである。沼野利佳准教授は、その壁を破る革新的な技術として、液滴エレクトロポレーション法を開発し、新法によるiPS細胞の量産化を手がけている。これは、細胞と導入したい山中因子(細胞の初期化を促す遺伝子)を封入した液滴に、電圧を印加することで細胞表面に開いたわずかな孔から遺伝子が導入されるという革新的な手法だ。従来のウイルス感染法に比べて、iPS細胞ががん化しにくく、少ない細胞で簡便に作製できるという。将来、移植に適応可能なiPS細胞の量産化を実現する新手法として注目が集まる。
ウイルスに頼ることなく、簡便にiPS細胞を作製する
京都大学の山中伸弥教授が、皮膚や血液などの体細胞に4つの因子を導入することで、細胞が初期化し、分化多機能性を持つiPS細胞になることを示して、2012年にノーベル生理学・医学賞を受賞したことはよく知られている。このiPS細胞を用いた再生医療は、失われた身体の機能や欠損を取り戻し、病気や怪我の根治を実現する革新的な医療として、世界中から大きな期待が寄せられている。
現在、このiPS細胞を用いた臨床研究が進められているが、実用化の障壁となっているのが、均質な性質を持つiPS細胞の作製の難しさと、実際にiPS細胞になる細胞が1%程度という、作製効率の悪さだ。
従来、iPS細胞の作製には、山中因子を細胞に導入するためにウイルスが用いられてきたが、これが原因で細胞ががん化し、腫瘍ができることがあった。ウイルス由来配列の残存などの危険性も指摘されている。そこで現在、ウイルスを用いずにiPS細胞を作製する方法が検討されているが、そうするとさらに作製効率が落ちてしまう。また、iPS細胞の作製・培養には熟練した専門技術者が必要なうえ、コンタミネーション(汚染)を防ぐための特殊な施設管理体制も必要になる。
「そこで、私たちが開発したのが、液滴エレクトロポレーション(電気穿孔法)によるiPS細胞の作製です。細胞膜は油の層でできているのですが、瞬間的に静電気ほどの数kV級の電圧をかけてやると、細胞膜が緩んで、微細な孔ができ、そこから山中因子を取り込むことができると考えられるのです。つまり、ウイルスを用いる必要はありません。
ところが、市販のエレクトロポレーション装置の場合、高い電圧の電気パルスを発生させるための高価なパルスジェネレーターが必要なうえ、これを用いると、半分以上の細胞が死滅してしまいます。そこで我々は、市販品と異なる直流電解を用いて、簡便に、高効率にiPS細胞をつくることができる、細胞にやさしい作製装置を開発しました」と、沼野准教授は説明する。
オンチップiPS細胞で量産化を狙う
そのしくみは、絶縁体である油相の中に、細胞と4つの山中因子を入れた数μlほどの液滴を垂らし、金属電極から直流電解をかけるというものだ。すると、球状の液滴が反応して、1分間に数百回ほど、プラス極とマイナス極の間を行ったり来たり、素早く往復運動を繰り返すようになる。
「水と油が混ざり合わない性質を利用しているわけですね。そして、最初の帯電で、液滴がたとえばマイナス極のほうに引き寄せられて接触すると、今度は極性がマイナスに反転して反発し、プラス極へ向かいます。これを何度も繰り返す中で、液滴が往復運動し、その間に微弱な電流が流れ、細胞膜が緩んで、山中因子が細胞の中に導入されるのです。しかも、反応場である液滴は数μl程度と小さいものの、この中に約1万個の細胞と山中因子である4種類の遺伝子が入っていて、効率よく遺伝子を細胞に導入することができます。液滴のまわりは油相で隔てられているため、反応場がコンタミネーションする心配もありません」(沼野准教授)
特筆すべきは、高い電圧をかけても液滴に流れる電流自体は非常に微弱で、細胞にはほとんど影響しないことにある。この液滴を採取して培養液に入れてiPS細胞を培養するまで、一連の工程には難しい作業は不要で、自動化もしやすいという特長がある。
「チップ状のマイクロ流路の中の数pl(ピコリットル)ほどの液滴でこの実験系を展開すると、さらに反応場が小さくなることで、遺伝子の拡散効率が高まり細胞により入りやすくなると考えられます。マイクロ流路は完全閉鎖系のためコンタミネーションの心配もありませんし、同時に山中因子を同じ細胞に導入することができ、短時間でiPS細胞を大量につくることができるようになるでしょう」
この液滴エレクトロポレーションの開発は、同大のバイオMEMS(Micro Electro Mechanical System)の研究者で、細胞穿刺用のマイクロニードルアレイの開発などを手がけてきた柴田隆行教授や、静電操作技術を生命科学へ応用されている栗田弘史助教らと共同で進めている。すでに特許を取得し、エレクトロポレーション装置を製造しているネッパジーン株式会社と共同で、社会実装に向けた検討も重ねている。
「現在、順天堂大学では、iPS細胞を使った再生医療としてパーキンソン病治療の臨床研究に取り組んでいますが、順天堂大学のゲノム・再生医療センター赤松和土先生に、私たちが作製したiPS細胞の機能分化の特性について調べていただいているところです。そのほか、京都大学、大阪大学、慶應義塾大学などでも、これまで治療が困難とされてきた神経の変性疾患に関する臨床研究が始まっています。こうした成果をできるだけ早く社会に還元できるよう、我々も量産化に向けた取り組みを加速させていきたいと思っています」
細胞をいっぺんに操作することで、病気の治療に役立てたい
ところで、沼野准教授自身がiPS細胞に関わるようになったのはここ数年のことだが、それまではおもに哺乳類の概日リズムを制御する研究を手がけてきた。
「地球上のすべての生物は24時間周期の概日リズムを刻んでいますが、これを支えているのが、20種類ほどある、時計遺伝子と呼ばれる遺伝子で、これらが脳の中で24時間周期で関連しながら機能しています。これは非常に強固なシステムで、数個の遺伝子変異が起こったくらいでは、簡単には壊れません。もし、時計遺伝子をコントロールしようとするなら、いっぺんにたくさんの遺伝子を操作する必要があるのです」と沼野教授は語る。
たとえば、日本から渡米した場合、目から入った光の刺激で、脳の時計遺伝子が影響を受けて、現地時間にリセットする調整を促す。しかし、体全体の時計を何時間も一気に動かすのは難しく、末梢組織の時計は脳の時計との同期に時間がかかるため、時差ボケに悩まされることになるのだという。
「そこで、遺伝子による概日リズム形成のメカニズムを解明し、それをいっぺんに調整することで、生理現象を変えられないか、たとえば時差ボケを一気に解消するとか、派生的に起こる病気の治療法に役立てられないかと考えてきました」
そうした中で出合ったのが、たくさんの遺伝子を一気に操る手段としてのエレクトロポレーションだった。また、この方法を活用すれば、がん細胞を特異的に攻撃するCAR(キメラ抗原受容体発現)-T細胞を改善するような遺伝子を入れて、がん治療に役立てることもできるかもしれない。
「この手法を発展させれば、iPS細胞だけでなく、さまざまな遺伝子操作にも活用できるでしょう。さらにどれくらいの遺伝子が入るのか、どういう種類の細胞なら機能調節ができるのかなど、研究を深めていきたいと考えています」と、沼野准教授は展望を語った。
(取材・文=田井中麻都佳)
取材後記
お父様は循環器内科、お母様は小児科のお医者様で、親戚を含めて医者一家の中で育った沼野准教授。大学病院に勤めていたお父様が家に帰ってくるなり、「今日は患者さんが亡くなった」と肩を落とす姿を見ることもあったという。
「医者になろうかとも思いましたが、親の姿を見るにつけ、病気を根本から治したり、予防したりすることができればと考えるようになり、基礎研究の道に進むことにしました」と沼野准教授は語る。
折しも、学生時代にヒトゲノム計画が始まり、より上流で病気を食い止める研究が進展しつつあった。ヒトゲノム計画を牽引していた日本の代表研究者、榊佳之前学長の研究室で博士の学位を取得し、その後、豊橋技科大へ。以後、10年に渡り、多様な研究者や企業とのコラボレーションにより、夢を実現しつつある。その笑顔は、明るくたおやかで、ベテラン医師のような安心感がある。今後のさらなる活躍に期待しています。
Researcher Profile

Dr. Rika Numano
Dr. Rika Numano received her M.S. degree in engineering and PhD degree in doctor in 1997 and 2001 respectively from University of Tokyo, Japan. Since she started her career at Toyohashi University of Technology, had been involved in the chronobiology, molecular biology, and neuroscience. She is currently an associate professor at the Department of Applied Chemistry and Life Science, Toyohashi University of Technology.
Reporter Profile

Madoka Tainaka is a freelance editor, writer and interpreter. She graduated in Law from Chuo University, Japan. She served as a chief editor of "Nature Interface" magazine, a committee for the promotion of Information and Science Technology at MEXT (Ministry of Education, Culture, Sports, Science and Technology).
ここでコンテンツ終わりです。
