
ここからコンテンツです。

Artificial enzyme for asymmetric synthesis using a synthetic chiral polymer
By Shinichi Itsuno
Cinchona alkaloid derivatives show catalytic activity in various kinds of asymmetric transformations in organic synthesis. These transformations are necessary steps in the production of pharmaceuticals. Shinichi Itsuno and his colleagues have successfully synthesized chiral polymers containing a cinchona alkaloid sulfonamide derivative as a repeating unit. This is the first example of a chiral polymer of cinchona sulfonamide, which shows high catalytic activity in the enantioselective desymmetrization of cyclic anhydrides.
Enzymes, high-molecular-weight chiral polymeric compounds, are complex biological catalysts. Capture of the substrate molecule, catalyzing the reaction, and release of the product are three important events performed by enzymes. In order to accomplish these events using a synthetic catalyst, the catalyst must necessarily have a large molecular weight so that it can act as a highly specific catalyst. To date no synthetic chiral polymers had been designed for this purpose, but now a research team from the Department of Environmental and Life Science at Toyohashi University of Technology has investigated a novel synthetic method for preparing chiral polymers containing repeating units of cinchona sulfonamide.
The lead author Shohei Takata said, "After testing many reaction conditions for the polymerization, we have synthesized chiral polymers containing cinchona sulfonamide repeating units. Chiral polymers are easily prepared according to the method we established."
"We have found that Mizoroki-Heck coupling was successful in synthesizing cinchona sulfonamide polymers," explains the leader of the research team, Professor Shinichi Itsuno, "Moreover, our chiral polymers showed high catalytic activity in asymmetric reactions.” Various kinds of such chiral polymers may be synthesized using this newly developed methodology to obtain various types of synthetic enzymes for specific reactions
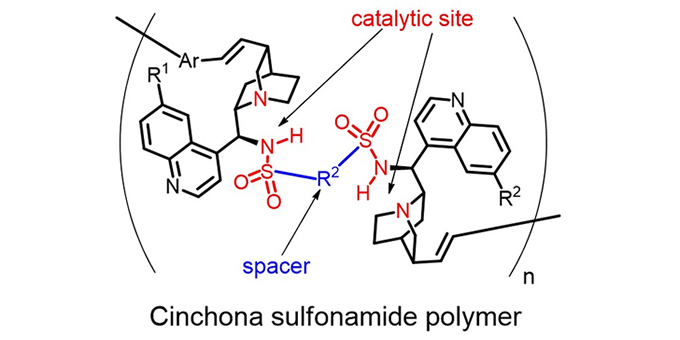
Furthermore, the chiral polymers developed in this study are insoluble in the usual organic solvents or water. The insoluble polymeric catalysts can be packed into a column, into which the substrate compounds can be introduced. The desired product can then be continuously obtained from the column. Without a usual reaction vessel, a continuous flow system may be possible using the polymeric catalyst. The flow system is a necessary technology for the automation of fine chemical syntheses.
Funding agency: This work was partly supported by a Grant-in-Aid for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No.2401) and Scientific Research (C) JSPS KAKENHI Grant Number JP15H00732, JP15K05517.”
Reference
Shohei Takata, Yuta Endo, Mohammad Shahid Ullah, and Shinichi Itsuno (2016). Synthesis of cinchona alkaloid sulfonamide polymers as sustainable catalysts for the enantioselective desymmetrization of cyclic anhydrides, RSC Advances, 6 (76), 72300-72305. 10.1039/C6RA14535C
不斉合成のための合成酵素の開発
高度な不斉反応触媒活性を有するシンコナアルカロイドスルホンアミド型高分子シンコナアルカロイド誘導体は、金属を使用しないキラル有機分子触媒として、様々な不斉反応の触媒として作用することが知られています。豊橋技術科学大学の研究グループは、スルホンアミド型シンコナアルカロイドを主鎖構造に組み込んだ合成高分子の新規合成法に成功しました。得られたキラルシンコナスルホンアミド高分子は高度な不斉触媒活性を示し、今後の合成酵素開発に大きく貢献する成果を得ました。
生体内で必要とされる様々な化学反応を行うために酵素が活躍しています。酵素は、タンパク質であり分子量の大きな巨大分子(高分子)化合物です。酵素は基質分子を間違いなく取り込み、反応を触媒し、生成物を放出する、という重要な働きを担っています。このような働きを実現するためには、触媒である酵素が巨大分子であることが極めて重要です。このような観点から、合成高分子であって高度な触媒活性を有する分子(合成酵素)の開発は、ファインケミカル合成分野で欠かせない技術であるはずですが、これまでにこのような合成高分子触媒の合成法そのものがほとんど研究されていませんでした。本研究では、一つの不斉反応に焦点をしぼり、その反応を効率よく触媒することのできるキラル高分子不斉触媒の開発に取り組みました。
シンコナアルカロイド誘導体が、多くの不斉反応の触媒として有効に働くことが知られているので、これを高分子の繰り返し単位として高分子主鎖構造中に組み込むことができれば、高分子不斉触媒のもっとも単純な形としてのキラル高分子を得ることができます。これまでにシンコナアルカロイド類の高分子化または高分子主鎖への組み込みついては報告例がありませんでした。本研究では、シンコナアルカロイドの持つ多彩な官能基を巧妙に利用して触媒活性部位を全く損なうことなく、高分子主鎖構造に組み込むことに成功しました。この高分子化のキーとなる反応は、溝呂木‐Heck反応です。溝呂木‐Heck重合法の開発により、初めてシンコナスルホンアミド型高分子を合成することができました。
得られたキラル高分子が触媒として働くかどうかが重要です。本研究では、実際に酸無水物の非対称化反応にこのキラル高分子を用いたところ、非常に高い触媒活性を示すことを明らかにしました。この高分子は通常の有機溶媒に溶けないため、反応後に回収することが極めて容易で、回収した高分子は触媒として何度でも使うことができます。将来的には、カラムに充填して原料化合物をその中に流すだけで、連続的に有用な生成物を連続的に取り出せるフローシステムへの応用が可能です。
本研究は、文部科学省・日本学術振興会科研費(JP15H00732, JP15K05517)の補助を受けて行われました。
Researcher Profile

| Name | Shinichi Itsuno |
|---|---|
| Affiliation | Department of Environmental and Life Sciences |
| Title | Professor |
| Fields of Research | Asymmetric reaction / Polymer-supported catalyst / Peptide folding |
ここでコンテンツ終わりです。
