
ここからコンテンツです。

Applying Micro-Electro-Mechanical Systems (MEMS) to medicine and drug discovery
Takayuki Shibata

Professor Takayuki Shibata is developing various devices for manipulating individual cells and analyzing cellular functions using Micro-Electro-Mechanical Systems (MEMS). Professor Shibata aims to apply MEMS to advanced medicine and drug discovery by constructing a platform that enables the diagnosis of individual cells as well as the regulation of cellular functions.
Interview and report by Madoka Tainaka
A micro device working on individual cells
Micro-Electro-Mechanical Systems (MEMS) refers to the technology whereby micromachining technology is used to fabricate miniaturized devices that integrate electrical and mechanical elements. MEMS came under the spotlight in 1987 when AT&T Bell Laboratories introduced micro gears with diameters ranging from 125 to 185μm at an international conference called Transducers held in Tokyo. Thereafter, the research and development of MEMS has been mainly led by the U.S., Europe, and Japan by using advanced micromachining technology that was developed from semiconductor technology. Nowadays, MEMS technology is used in various devices including acceleration sensors in automobile air bags and smart phones, the inkjet nozzle of printers, the mirror and switch of optic devices and analysis equipment in the field of life sciences.
“Great expectations are placed on MEMS to become a key technology for innovation since its range of potential applications is quite broad. On the other hand, the MEMS technology demanded by each field is different, and therefore MEMS research is progressing in various unique ways adapted to suit the needs of each field. Although we talk about MEMS in general, it actually represents quite diverse fields, and is also constantly evolving through trial and error,” says Professor Takayuki Shibata.
Professor Shibata is applying cutting edge MEMS based research to construct very tiny tools that work on a biological cell, which is the smallest unit of life. “The size of a cell is only 10 to 20μm. Up to now there have been no tools that could handle both individual cells and all the cells at once. If our technology is put into practice, we expect that researchers will be able to control and analyze a large number of cells simultaneously, leading to new findings in life science. Not only that, we would be happy if our technology can contribute to facilitating an acceleration in the process of drug discovery and medicine,” Professor Shibata says.
The applications of MEMS in the medical field have mushroomed since the complete decipherment of the human genome in 2003. More recently their practical implementations have been increasing. In this context, Professor Shibata has received attention by developing a microneedle array for pricking cells - 600,000 pcs of tiny hollow needles made of glass with an outer diameter of 5.6μm and an inner diameter of 3.2μm arrayed on a 1cm2 size microchip.
“This needle array is an innovative device that can simultaneously introduce DNA molecules and extract biomolecules into and from 600,000 individual cells . Conventional methods for cell analysis can only provide information on the average value of all the cells since their analysis equipment checks all cell fracture extracts in a large quantity. On the other hand, if we can work on individual living cells in the scale of one million and analyze them, highly accurate statistical analysis will become possible, even as far as understanding the individuality of each cell. Furthermore, since the diameter and spacing of the needles is adjustable in MEMS, we can create various designs depending on its application.”
Using electrical driving force and mechanical oscillation to introduce DNA into cells
To fabricate a needle array, one must first make a circular pattern on silicon substrate using a method known as photolithography and then vertically etch fine holes into the substrate. The next step is to make them react with the water and silicon by exposing them to high temperature steam at 1000°C to form a glass film (SiO2) in the inner wall of the hole. Finally it is necessary to remove the silicon using an alkaline solution to create a minute glass needle.
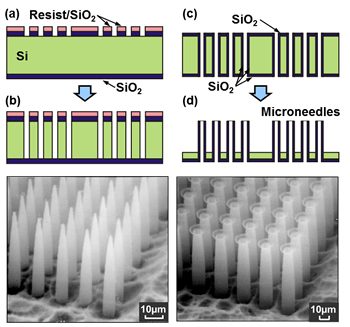
“This technology is used in the manufacturing of semiconductors, and the location, shape, height and aperture of the needles can be freely modified. Using these characteristics, we can make even thinner needles or a type of tube with its tips spread out like an octopus’s suction cup. Actually, the suction cup tube was created by accident, but once I observed it I realized that it could be used as a manipulator for creating desirable cell patterns and three-dimensional structures by grabbing cells and lining them up freely,” Professor Shibata explains.
If this technology can work on individual cells, the biochemical reaction of a large number of cells under the same conditions can be observed. This technology can also be applied to comprehensively study the differences in reaction based on variations in the conditions. Much is expected of its potential applications in regenerative medicine and genome drug discovery. However, there still exists one significant obstacle - it is very difficult to prick a small cell with a needle without causing any deformation.
“Cells are very small and delicate, so if we try to forcibly prick one with a needle, its cellular membrane may be punctured and destroyed, and the cell would eventually die. You might have seen a microscopic image of pricking an egg cell with a glass needle in IVF (In Vitro Fertilization), but almost all types of cells (so-called somatic cells) that make up our body are only one tenth of the size of the egg cell so it is very difficult to prick them by sucking with a needle under negative hydrostatic pressure. In addition, if you try to introduce a solution into an array of needles by the application of external pressure, but the aperture of needle is too small, the solution would seek whichever individual needle offered the least hydraulic resistance, and only be released from that point. No matter how sophisticated our needle processing, it is impossible to fabricate all needles to precisely the same size.”
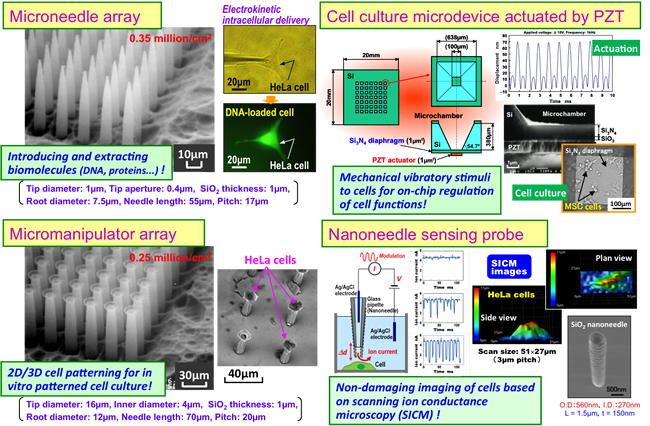
To address this problem, Professor Shibata adopted a method using an electric field and mechanical oscillations when penetrating cell membranes and introducing DNA into a cell with a needle. DNA is negatively charged in a solution so if the potential inside the cell is rendered positive by applying appropriate voltage, DNA can be injected into the cell through the tip of the needle. Furthermore, it becomes easier to penetrate cell membranes without causing serious cell damage when mechanical oscillation is applied during the insertion process with a needle. This is due to an increase in the cell’s viscous resistance.
“Think about the suspension in automobiles. It is mechanically composed of a combination of springs and dampers. Springs can be deformed proportional to the applied force. On the other hand, dampers can also be deformed when pressed slowly just like the spring; however, if the damper is pressed quickly, it becomes hard and difficult to deform. It becomes easier to penetrate a cell with a needle, as with the damper, when one applies oscillations to quickly deform its cell membrane. This technology was made possible by applying my knowledge acquired from mechanical engineering and cell-capture, in other words by understanding its mechanical characteristics.”
Application to mass production of iPS cells and genome editing
Moreover, Professor Shibata is aiming to construct a platform for analyzing and manipulating cells by handling various technologies such as hollow microneedle arrays, suction cup type manipulator arrays, piezoelectric type micro cell medium devices, nanoneedle-based sensing probes for capturing dynamic changes of cells, and multi-functional scanning bioprobe microscope with hollow nanoneedle.
“At this point, we are only preparing the necessary tools for analyzing and manipulating cells, but we want to apply these technologies for the mass production of iPS cells in the future. Currently, iPS cells are made manually by skilled personnel, but the success rate of creating iPS cells is only 1%, which is very low in efficiency. If we can use our needle array to establish automatic mass production of iPS cells, the application of iPS cells will progress even further, and it will become more convenient to elucidate the mechanisms of diseases and to study the effects and toxicity of various drugs.”
Professor Shibata says that he wants to accelerate the process of finding practical applications for his work by collaborating with medical institutions. His will continue to strive to develop fundamental technologies for advanced medical research.
Reporter's Note
Professor Shibata, as a former researcher in precision engineering, used to produce diamond thin films and three-dimensional structures for use in devices. In the meantime, he became interested in the mystery of life and turned to the field of BioMEMS research after being involved with the production of microchips for amplifying DNA and analyzing blood. He feels that it is his mission to apply the technologies that he learned in the field of manufacturing, where reproducibility is emphasized, to the progress of life science.
“It is not well known but Toyohashi University of Technology has the one of the best cleanrooms in the world. I am very fortunate therefore to enjoy a research environment which is not only optimum for creating MEMS, but is also supported in such a way that active collaboration with researchers in different fields is made easy. As it happens, my interest in the mass production of iPS cells emerged from a conversation with a researcher in a different field,” Professor Shibata says. It is greatly hoped that this MEMS technology, originating from Toyohashi, will contribute to rapid advancements in medicine and drugs around the world.
微細加工技術(MEMS)を医療や創薬へ役立てる
MEMS(Micro Electro Mechanical System=マイクロ電子機械システム)を活用して、一つひとつの細胞を操作したり、細胞の機能を解析したりするためのさまざまなデバイスの開発を手がける柴田隆行教授。MEMSにより個々の細胞の診断や機能の制御を可能にするプラットフォームを構築し、先端医療や創薬に役立てたいという。
一つひとつの細胞に作用する微小なデバイス
MEMS(Micro Electro Mechanical System)とは、微細加工でつくられる超小型の電子機械のこと。1987年に東京で開催されたトランスデューサーという国際会議において、AT&Tベル研究所が直径125〜185μmほどのマイクロ歯車を発表したのを機に、脚光を浴びるようになった。以後、半導体技術で培った微細加工を得意とする欧米や日本を中心に研究開発が進められ、いまやMEMSは、自動車のエアバックやスマートフォンに搭載されている加速度センサをはじめ、プリンターのインクジェットのノズル、光デバイスのミラーやスイッチ、生命科学分野の分析装置など、じつにさまざまな機器に使われている。
「それだけMEMSは応用の幅が広く、イノベーションのキーテクノロジーとして大きな期待が寄せられているのです。一方で、分野ごとに求められるMEMS技術は違っていて、それぞれ独自のアイデアで研究が進められています。つまり、一言でMEMSと言ってもじつに多彩で、それぞれ試行錯誤を重ねながら進化しているのです」、と柴田隆行教授。
その柴田教授が取り組むのは、MEMSを活用して、生命の最小単位である細胞に働きかけるための極めて小さな道具をつくるという、じつに先進的な研究である。「細胞の大きさは10〜20μmほどしかなく、これまで一つひとつの細胞に作用し、かつ、たくさんの細胞をいっぺんに扱うような道具というのは存在しませんでした。これが実現すると、多数の細胞を同時に操作したり分析したりすることが可能になり、生命科学の新しい発見につながると期待しています。ひいては、創薬や医学の飛躍的な進展に貢献できれば嬉しいですね」と柴田教授は語る。
MEMSの医療分野への応用は、2003年にヒトゲノムの完全解読を受けて本格化し、まさにいま、実用化に向けて競争が加熱している。そうした中、柴田教授は、ガラスでつくった外径5.6μm、内径3.2μmという微細な中空の針を、1cm2のマイクロチップ上に60万本も並べた細胞穿刺用のナノニードルアレイを開発して注目されているのだ。
「このニードルアレイは、一度に60万個の細胞それぞれからDNAを取り出したり、溶液を注入したりできる画期的なデバイスです。これまでの細胞分析というと、大量の細胞を破砕して抽出した物質をチェックしていますので、それでは細胞全体の平均値しか得られません。これに対して、100万個単位の生きた細胞一つひとつに働きかけ、分析できれば、細胞の個性までも理解した精度の高い統計処理が可能になる。しかも、MEMSでは針の太さや間隔を自在に変えられるので、用途に合わせてさまざまにデザインすることが可能なのです」。
電場駆動力と振動で、細胞へDNAを注入する
ニードルアレイを作製するには、まずシリコンの板にフォトリソグラフィという方法で円形のパターンをつくり、エッチングにより微細な穴を垂直に深堀りする。その後、1000度ほどの高温の水蒸気にさらすことで水とシリコンを反応させ、穴の内壁にガラスの膜(SiO2)を形成。最後に、アルカリ溶液を用いて、Siだけを除去することでガラス製の微細な針を作製するのだという。
「これは、半導体の製造に採用されている技術で、自在に場所や形、高さ、開口部の大きさなどを変えることができます。こうした特性を生かして、より細い針をつくったり、タコの吸盤のように先端が横に広がったタイプのチューブを作製したりしています。じつは吸盤タイプのものは失敗の産物なのですが、細胞をつかんで自在に並べることで、目的とする細胞のパターンや立体構造をつくるマニピュレータとして使えるとひらめきました」、と柴田教授は言う。
無数の細胞に、一つひとつ働きかけることができれば、同じ条件下で大量の細胞の反応を見ることができ、網羅的に条件を変えて反応の違いを調べるといった実験にも活用できるとして、再生医療やゲノム創薬などへの応用に期待が高まる。しかし、課題もある。微細な細胞に針を刺すのは、それほど簡単ではないのだ。
「じつは細胞は小さく繊細なので、無理に針を刺そうとすると細胞膜が破れたり潰れたりして、死んでしまうんですね。人工授精などの際に、卵細胞にガラスの針を刺す顕微鏡の映像をご覧になったことがあると思いますが、私たちの身体をつくっている通常の細胞は卵細胞の10分の1ほどの大きさしかなく、針をそのまま押し付けて刺すのは困難です。しかも、針が細すぎると圧力をかけて溶液を注入したくても、一番抵抗の低い針からしか出ない。いくら精密に加工をしても、すべての針の大きさを完璧に揃えるのは不可能ですからね」。
ここで柴田教授が採用したのが、針を刺す際に電場と振動を利用するという方法だ。溶液に入れたDNA分子は負の電荷を帯びているため、細胞側がプラスになるように電圧をかけてやると針先から細胞内にDANが導入できるようになる。さらに、針の先端を細胞に少し押し込んだ状態で、ごく微小な振動をかけて細胞膜にかかる応力をコントロールしてやると、針が細胞を押しつぶすことなく、生きたままの状態で刺すことができるという。
「車のサスペンションをご存知ですよね。機械要素としてバネとダンパーが組み合わされたものです。バネは力を加えるとその大きさに比例して変形します。一方、ダンパーはゆっくり押すとバネと同じように変形しますが、速く押そうとすると変形しにくくなり硬くなります。細胞もこのダンパーのように振動をかけて細胞膜を速く変形させようとすると硬くなり、針が刺しやすくなるという原理です。機械工学で学んだ知見を生かし、細胞を材料として捉えて、その機械的性質を理解することで実現した技術です」
iPS細胞の量産化やゲノム編集へ活用
さらに柴田教授は、こうしたミクロの中空のニードルアレイや吸盤型のマニュピュレータアレイ、圧電駆動型のマイクロ細胞培養デバイス、細胞の動的形態変化などを捉えるナノニードル計測プローブ、さらには中空ニードルを応用した多機能走査型バイオプローブ顕微鏡など、さまざまな技術を取り揃えることで、細胞の分析・操作のためのプラットフォームづくりを目指している。
「現段階では、細胞の分析・操作に必要な道具を揃えているところですが、将来的にはiPS細胞の量産化にこの技術を役立てたいと考えています。というのも、現状はiPS細胞の作製は熟練者が手作業で行っているのですが、その方法では、iPS細胞として樹立するのは1%程度と効率が悪いのです。ニードルアレイを使ってiPS細胞を自動的に量産できれば、iPS細胞の活用が進み、疾患のメカニズムを解明したり、薬の効能や毒性を調べたりといったことが、もっと手軽にできるようになるでしょう」
今後は医療機関との共同研究を進め、実用化に向けて研究を加速させていくという柴田教授。先端の医療研究の基盤技術の構築に向けた挑戦は続く。
(取材・文=田井中麻都佳)
取材後記
もともと柴田教授は、精密工学の研究者として、デバイスへの応用を念頭に、ダイヤモンドの薄膜や立体構造の製作を手がけていた。一方で、以前から生命の不思議に興味を持ち、留学先の英国やスイスで、DNAを増殖したり血液を分析したりするマイクロチップの作製に携わったことから、バイオMEMSの研究へ転向。再現性を重視するモノづくりの世界で培った技術力を、生命科学の発展に生かすことが自らの使命だと感じている。
「じつは豊橋技科大には、世界でも有数のクリーンルームがあり、MEMSの作製においてこれほど恵まれた環境はありません。しかも、異分野の研究者との交流も盛んで、コラボレーションがしやすい。じつはiPS細胞の量産化の話も、そうした異分野の研究者との交流から始まったんですよ」と柴田教授。この豊橋から、医療や医薬を飛躍的に進展させるようなMEMS技術が花開くことに大いに期待したい。
Researcher Profile

Dr. Takayuki Shibatareceived the BS, MS, and PhD. degrees in precision engineering from Hokkaido University, Sapporo, Japan, in 1987, 1989, and 1999, respectively. Following two years with Sumitomo Electric Industries, Ltd., he was with the Faculty of Engineering, Hokkaido University, from 1991 to 2001, and the Faculty of Engineering, Ibaraki University, from 2001 to 2005. Since 2005, he has joined the Faculty of Engineering, Toyohashi University of Technology. He is currently working as a professor in the Department of Mechanical Engineering, Toyohashi University of Technology, from 2007.
Reporter Profile

Madoka Tainaka is a freelance editor, writer and interpreter. She graduated in Law from Chuo University, Japan. She served as a chief editor of “Nature Interface” magazine, a committee for the promotion of Information and Science Technology at MEXT (Ministry of Education, Culture, Sports, Science and Technology).
ここでコンテンツ終わりです。
