Research highlights
New Method for the Construction of Quaternary Chiral Carbon Centers
Pharmaceutical and agricultural chemistry has seen a dramatic growth in the importance of chiral technology for producing enantiomerically pure molecules over the last two decades. In particular, there are strong demands for the development of methods for the highly enantioselective formation of quaternary chiral carbon centers.
Alkyl chlorides are well recognized as useful synthetic intermediates because of the ability of chlorine atoms to be removed. Once alkyl chlorides having a chlorinated chiral carbon center are synthesized in a highly enantioselective manner, the compounds can be converted into various chiral molecules via SN2 substitution while maintaining their enantiomeric purity. However, this process has not been applied successfully for the construction of quaternary carbon centers because tertiary halides rarely undergo SN2 reactions as described in organic chemistry textbooks.
Here, Kazutaka Shibatomi and colleagues at Toyohashi University of Technology have achieved the highly enantioselective chlorination of a wide range of active methine compounds and subsequent amazingly unimpeded SN2 substitution at the tertiary carbon.
A novel chiral oxazoline ligand 1 having the spiro backbone was synthesized and its copper(II) complex was used for the enantioselective chlorination of β-ketoesters. The reactions successfully produced the desired α-chloro-β-ketoesters with excellent enantioselectivity (up to 98% ee). Nucleophilic substitution of the resulting chlorides proceeded smoothly resulting in a variety of chiral molecules such as α-aminoesters, α-(alkylthio)esters, and α-fluoroesters, without loss of enantiopurity.
This method yields a variety of chiral molecules having a quaternary chiral carbon center. An advantage of this method is that it allows for the formation of multiple optically active compounds from a single intermediate.
- Reference:
- Kazutaka Shibatomi, Yoshinori Soga, Akira Narayama, Ikuhide Fujisawa, and Seiji Iwasa.
- Highly Enantioselective Chlorination of β-Keto Esters and Subsequent SN2 Displacement of Tertiary Chlorides: A Flexible Method for the Construction of Quaternary Stereogenic Centers.
- Journal of the American Chemical Society 134, 9836 (2012).
- DOI: 10.1021/ja304806j
- Department of Environmental and Life Sciences, Toyohashi University of Technology.
- http://www.tutms.tut.ac.jp/STAFF/SHIBATOMI/index.html.en
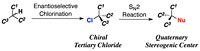
Synthetic Strategy for the Construction of Quaternary Chiral Carbon Centers.
Enlarge Image
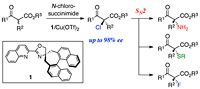
Enantioselective Chloriation of β-Ketoesters and Subsequent SN2 Reactions.
Enlarge Image

Kazutaka SHIBATOMI
Enlarge Image
4,4,4-Trifluorocrotonaldehyde: A Versatile Building Block for the Synthesis of Chiral Trifluoromethyl Compounds
Selective incorporation of trifluoromethyl groups into organic molecules is an important in drug discovery. Even though a large number of pharmaceuticals contain trifluoromethyl group(s), developing flexible methods for the construction of trifluoromethylated chiral carbon centers in a high enantioselective manner remains a challenging task.
Since α,β-enals have been widely used as precursors for numerous organocatalytic asymmetric transformations, it is envisaged that 4,4,4-trifluorocrotonaldehyde 1 would be a versatile precursor for the construction of trifluoromethylated chiral carbon centers. However, there is no report on the isolation of 1 or its use in asymmetric transformations. One possible reason for this is the high volatility of 1 and the consequent difficulty in its purification.
Here, Kazutaka Shibatomi and colleagues at Toyohashi University of Technology have succeeded in the isolation of this aldehyde for the first time and have succeeded in its application to the organocatalytic asymmetric 1,4-addition with several types of nucleophile.
4,4,4-trifluorocrotonaldehyde 1 was synthesized by the oxidation of 4,4,4-trifluoro-2-butenol 2 with manganese dioxide. After the removal of manganese dioxide by filtration, the filtrate was distilled to afford nearly pure 1 in 47% yield. The organocatalytic 1,4-addition of 1 with several nucleophiles such as heteroaromatics, alkylthiols, and aldoximes afforded the corresponding products bearing a trifluoromethylated chiral carbon center with high enantiopurity. A resulting product was converted into an MAO-A inhibitor, befloxatone.
The present method provides a powerful method for the preparation of optically active trifluoromethyl compounds.
- Reference:
- Kazutaka Shibatomi, Akira Narayama, Yoshiyuki Abe, and Seiji Iwasa.
- Practical Synthesis of 4,4,4-Trifluorocrotonaldehyde: A Versatile Precursor for the Enantioselective Formation of Trifluoromethylated Stereogenic Centers via Organocatalytic 1,4-Additions.
- Chemical Communications 48, 7380 (2012).
- DOI: 10.1039/C2CC32757K
- Department of Environmental and Life Sciences, Toyohashi University of Technology.
- http://www.tutms.tut.ac.jp/STAFF/SHIBATOMI/index.html.en
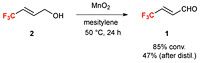
Synthesis of 4,4,4-Trifluorocrotonaldehyde.
Enlarge Image
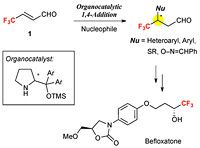
Organocatalytic 1,4-Additions of 4,4,4-Trifluorocrotonaldehyde and its Application to the Synthesis of Befloxatone.
Enlarge Image