Research highlights
A Simple Approach to Chiral Trifluoromethyl Compounds
Organofluorine compounds have attracted significant attention in drug discovery. For instance, introduction of a trifluoromethyl group into biologically active compounds often modifies their physical and/or biological properties such as lipophilicity, metabolic stability, and bioavailability. Although the synthetic method for trifluoromethyl compounds has been progressing steadily, developing an efficient method for the construction of trifluoromethylated stereogenic center remains challenging task.
Diels–Alder reaction using trifluoromethyl olefins as the dienophile is known to be a useful method for the preparation of various trifluoromethyl compounds. However, there is no published report on the enantioselective version of this reaction; despite the fact that the reaction would efficiently provide the chiral cyclohexenes having a trifluoromethylated stereogenic center.
Now, Kazutaka Shibatomi and colleagues at Toyohashi University of Technology have succeeded in the enantioselective Diels–Alder reaction of β-trifluoromethylacrylates to give corresponding cyclohexenes having a trifluoromethyl group at the chiral carbon center. The resulting cyclohexenes could be converted into potential synthetic intermediates for new drug candidates.
The reaction of ethyl 4,4,4-trifluorocrotonate (1 in Fig.1) with cyclopentadiene or furan in the presence of chiral Lewis acid catalyst (2 in Fig.1), which was prepared from an oxazaborolidine and SnCl4, successfully afforded the desired cyclohexenes (3 in Fig. 1) with excellent enantioselectivity (99% ee). A resulting adduct was further converted into ethyl 6-trifluoromethyl-shikimate by a four-step sequence, which could be a key intermediate for anti-influenza agents.
The present method provides a powerful method for the preparation of optically active trifluoromethyl compounds, which will be helpful in new drug design.
- Reference:
- Kazutaka Shibatomi, Fumito Kobayashi, Akira Narayama, Ikuhide Fujisawa, and Seiji Iwasa.
- A Diels–Alder approach to the enantioselective construction of fluoromethylated stereogenic carbon centers
- Chemical Communications 48, 413–415 (2012).
- DOI: 10.1039/c1cc15889a
- Affiliation(s): Department of Environmental and Life Sciences, Toyohashi University of Technology.
- Website: http://www.tutms.tut.ac.jp/STAFF/SHIBATOMI/index.html.en
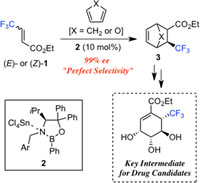
Fig.1 Enantioselective Diels-Alder Reaction of 4,4,4-Trifluorocrotonates.
Enlarge Image

Kazutaka Shibatomi
Enlarge Image