
ここからコンテンツです。

Free Architectural Design and Synthesis of Nanocomposites
Hiroyuki Muto

In recent years, nano-sized materials have been attracting tremendous attention in many research fields such as structural materials, cosmetics and medicine. The reason for this is that the unique physical properties of nanomaterials, such as quantum, surface and interface effects, facilitate the development of many new applications for these materials. Until now however, nanomaterials’ complexity hindered their effective utilization; resulting in little progress in their practical applications. Nevertheless, Dr. Hiroyuki Muto, professor of Institute of Liberal Arts & Science and Department of Electrical and Electronic Information Engineering (acting), had developed a simple but novel method that enables the synthesis of uniform nano-sized composite materials utilizing electrostatic interactions. Currently, Dr. Muto is advancing his research towards the mass production of nanomaterials.
Interview and report by Madoka Tainaka
The key is “electrostatic interaction”
“I think many of you know very well what a carbon nanotube is. However, if I ask you about the practical applications of a carbon nanotube, I doubt many of you would be able to answer my question, which would be fair enough, as few practical applications currently exist.
A carbon nanotube is a thread-like material that can conduct electricity and upon mixing with translucent materials, it can be utilized in many applications such as touch panels and solar panels. However, during preparation, carbon nanotubes tend to shrink into a ball of thread or harden upon mixing. Moreover, as it is difficult to obtain a uniform mixture of carbon nanotubes, a large amount is usually required during the mixing process. As carbon nanotubes are very expensive, it is not therefore easy to practically fulfill their immense theoretical potential.,” explains Dr. Muto.
In the conventional fabrication of composite materials, the required raw materials are mechanically stirred in a container with sticks or hard balls, which is also known as ‘mixing’. In reality, it is actually very difficult to obtain a very well balanced and distributed mixture. Despite the adoption of various measures to improvise the mixing process such as chemical additives and controlling the level of force and time during mixing, crucial issues such as structural decomposition resulting from the impact of mixing remained unresolved. Therefore, it is almost impossible to synthesize minute/fine composites by just mixing the materials.
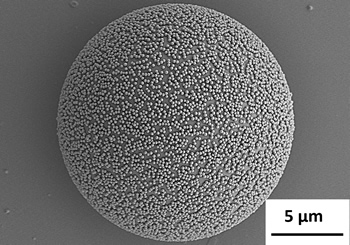
“When materials with different specific gravities are mixed, they tend to separate and resist uniform mixing. It is also a challenge to mix the proper amount of conductive materials (as mentioned previously for carbon nanotubes) and at the same time align them in a continuous arrangement. Therefore, we came up with the idea of synthesizing a composite material by adhering nano-sized materials around a micro particle that is used as the parent material (matrix).”
The principle of adhering the nano-sized materials onto the matrix particle is based on “electrostatic interaction.” Electrostatic interaction is the force that acts between positive and negative charges. Dr. Muto has succeeded in using this well-known physical phenomenon to construct composite materials in a simple manner.
Synthesizing a wide variety of composite materials by freely controlling the surface electric charges of a particle
This method for synthesizing a composite material is very simple and easy to understand.
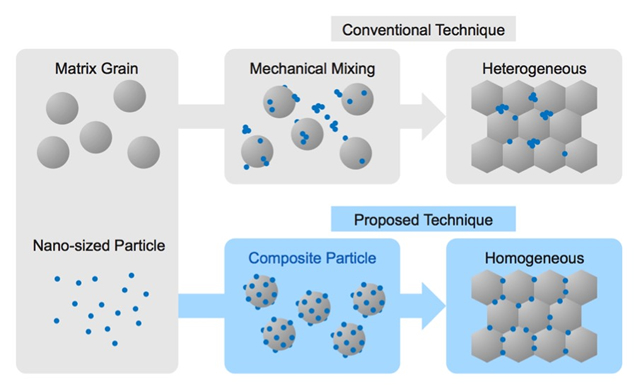
“First, the electric charges on the surface of the matrix particle and the nanomaterial (which is the additive) are controlled to be either positive or negative, in order to generate opposite polarities. Then, both materials are added into a solution such as water and stirred in such a way that the nanomaterial is successfully absorbed onto the surface of the matrix particle. The preparation procedure involves the addition of the materials into a polyelectrolyte solution (a material that produces polymer ions upon dissolution in water, such as the positively charged poly diallyldimethylammonium chloride or the negatively charged sodium polystyrenesulfonate) so that the surface of each material has either a positive or negative charge. This method enables quick room-temperature synthesis of composites, without being affected by the specific gravity difference of the constituent materials. Moreover, the amount of nanomaterials absorption can be freely regulated”, Dr. Muto explains.
Firstly, all materials have their own specific electric charge. For example, if silica (silicon oxide) is added into neutral water, it would become negatively charged. However, this does not always occur, and in some circumstances, the electric charge may be too weak and cannot be used as it is. However, Dr. Muto explains that if a polyelectrolyte is used, it is possible to charge the material uniformly with a strong electric charge. In addition, the alteration of electric charge between positive and negative is possible regardless of the original surface electric charge of the particle. By adopting this method, it is feasible to synthesize composites with various functionalities depending on the combinations of matrix particles and nanomaterials that are absorbed.
“Actually, this method is based on electrostatic interaction, which is already used in the synthesis of functional “nano” thin films (two-dimensional structures). So although the method is not new, we succeeded in using it for the first time to synthesize integrated three-dimensional architectural nanocomposites that consisted of particles of various sizes. When the composite is dried, it is applicable as a powder. By increasing the concentration, it can be utilized as a paste. In powder metallurgy, the composites can also be pressed, formed, and hardened thermally into a block. One of the highlights of this method is the ability to synthesize and design the dimension or form of the nanomaterials in order to utilize their properties in accordance to the specific requirements”, Dr. Muto proudly explains.
The composite particles synthesized in this way are bound strongly, which makes the material stable and more energy efficient compared to those synthesized by mechanical mixing. This method was not originally suitable for materials that did not possess wettability, water solubility or buoyancy because this process involves dissolution into a solvent. However, through the application of many creative ideas, it has become applicable for almost all materials at this point.
As an example, Dr. Muto worked on the development of a conductive material using carbon nanotubes. In this project, Dr. Muto succeeded in synthesizing a material that has high transparency, while greatly reducing the amount of carbon nanotubes used.
Working on the development of a manufacturing process towards mass production
Owing to the superiority of this research, it was adopted as one of the Industrial Technology Research Grant Programs of the New Energy and Industrial Technology Development Organization (NEDO) from 2009 to 2012. From 2015, the research has advanced further into the Innovative Design and Production Technology project in the Cross-ministerial Strategic Innovation Promotion Program (SIP) of the Cabinet Office. Currently, Dr. Muto is working on the process development that enables mass production of composite nanomaterials (composite particles). Moreover, industries in various fields are expressing their interest in this technology.
In relation to his goal, Dr. Muto said, “Material study does not immediately come across as a glamorous field of study, but in fact it has great influence on society. For example, just by changing the composition of a material, its strength can be doubled or reduced to half. It is therefore challenging. The production rate varies for different materials, but in the near future, I hope to be able to produce about 10 kg of composite particles per hour.” It is very exciting to see what kind of novel materials will be synthesized with this method developed by Dr. Muto.
This study was supported by Cross-Ministerial Strategic Innovation Promotion Program (SIP) of Council for Science, Technology and Innovation (CSTI), Japan.
Dr. Muto and his research colleagues were awarded the “Editor-in-Chief's Featured Article” by the Journal of Supercritical Fluids (Elsevier)

This is an award given to the top three papers chosen from all published articles in 2014 by the editorial committee of the Journal of Supercritical Fluids.
Awarded to
Kiyoshi Matuyana, Yu-ki Maeda, Takaaki Matsuda, Tetsuya Okuyama (National Institute of Technology, Kurume College) . Hiroyuki Muto (Toyohashi University of Technology)
Title of awarded article
Formation of poly (methyl methacrylate)-ZnO nanoparticle quantum dot composites by dispersion polymerization in supercritical CO2.
By TUT Research editor
Reporter's Note
Toyohashi University of Technology has many members who graduated from a technical college, as the university promotes collaborations with other technical colleges. Dr. Muto himself has also graduated from the National Institute of Technology, Fukushima College.
“People from technical colleges are accustomed to experiments, and many of them enjoy the process. Therefore, they share the tendency of trying out their ideas hands-on. This research started in the same way too, where I tried my idea in an experiment and surprisingly, the result was better than expected.” Dr. Muto laughed.
Dr. Muto was originally working on research regarding the fracture mechanics of materials. He chanced upon his current research when he first started to synthesize his own ceramics for evaluation purposes. It’s safe to say that Dr. Muto’s significant research results are the fruit of his dedicated attitude toward experiments.
Reference
- Matsuyama, K., Maeda, Y., Matsuda, T., Okuyama, T., and Muto, H. (2015). Formation of Poly(Methyl Methacrylate)-ZnO Nanoparticle Quantum Dot Composites by Dispersion Polymerization in Supercritical CO2, The Journal of Supercritical Fluids, 103, 83-89.
- Phuc, N. H. H., Okuno, T., Hakiri, N., Kawamura, G., Matsuda, A., and Muto, H. (2014). Synthesis of High-Edge Exposure MoS2 Nano Flakes, Journal of Nanoparticle Research, 16(1), 2199.
- Tan, W. K., Razak, K. A., Lockman, Z., Kawamura, G., Muto, H., and Matsuda, A. (2014). Synthesis of ZnO Nanorod-Nanosheet Composite via Facile Hydrothermal Method and their Photocatalytic Activities under Visible-Light Irradiation, Journal of Solid State Chemistry, 211, 146-153.
- Phuc, N. H. H., Okuno, T., Matsuda, A., and Muto, H. (2014). Ex Situ Raman Mapping Study of Mechanism of Cordierite Formation from Stoichiometric Oxide Precursors, Journal of the European Ceramic Society, 34(4), 1009-1015.
ナノ複合材料を微細に、自在にデザインする
近年、構造材料から化粧品、新薬にいたるまで、ありとあらゆる分野において、ナノサイズの物質が注目されている。 その理由は、「量子効果」や「表面・界面効果」といった、ナノ物質特有の物性により、材料に新しい機能を付加できる点にある。しかし、実際にはナノ物質を使いこなすことは難しく、実用化はさほど進んでいない。そうした中、武藤浩行教授は、「静電相互作用」を利用して、ナノサイズの複合材料を均質に、しかも自在にデザインする手法を開発。量産化へ向けて、研究を加速させている。
カギは「静電相互作用」にあり
「皆さん、カーボンナノチューブはよくご存知だと思いますが、実際に何に使っているかというと、すぐに思いつかないのではないでしょうか。それもそのはずで、じつは、まだほとんど実用化には至っていないのです。
カーボンナノチューブは糸状の電気を通す物質で、透明な物質に混ぜれば、タッチパネルや太陽光パネルなど、さまざまな用途に使うことができます。しかし、いざつくるとなると、物質とまぜる際に糸鞠状にかたまってしまったり、均質に適量を材料に入れることが難しかったりして、現状は大量に混ぜなければなりません。とても高価な物質ですから、なかなか実用化に結びつかないのです」と武藤教授は説明する。
従来、複合材料をつくる際に、多くの場合、容器の中で棒や硬い球などを用いて機械的に撹拌する、つまり「混ぜる」という手法がとられてきた。しかし物質を均質に混ぜるのは容易ではない。物質同士が混ざりやすいように薬品を使ったり、撹拌する強さや時間を変えたりするなど、さまざまな工夫が凝らされてきたが、混ぜる際の衝撃で物質の構造が壊れてしまうなど、いくつもの課題があった。そもそも、ただ混ぜるだけでは、複合材料を微細にデザインすることは難しい。
「当然、比重が違うものを混ぜると分離してうまく混ざりませんし、さきほどのカーボンナノチューブのように、導電性の材料を適量入れて、連結するように配置することも困難です。そこで我々が思いついたのが、母材(マトリックス)となるミクロの粒子のまわりにナノ物質をぺたぺたと貼付けて複合材料をつくるという方法です」
このとき、マトリックス粒子にナノ物質を吸着させる役割を担うのが「静電相互作用」である。静電作用とは、正(プラス)と負(マイナス)の電荷の間に働く引力のこと。この一般的にもよく知られた物理現象を利用して、武藤教授は複合材料を簡便につくることに成功したのである。
粒子の表面電荷を自在にコントロールし、多種多様な複合材料をつくる
そのつくり方は、じつに単純明快だ。
「マトリックス粒子の表面の電荷と添加物となるナノ物質の表面電荷を、それぞれプラスとマイナス、すなわち相反するように制御しておいて、その二つの物質を水などの溶液の中に入れて、ぐるぐるかき混ぜて吸着させるだけです。そのための前処理として、それぞれの物質の表面がプラスかマイナスの電荷を帯びるように、高分子電解質(水中で解離して高分子イオンとなる物質)の溶液(例えば正電荷を持つポリジアリルジメチルアンモニウムクロリドや負の電荷を持つポリスチレンスルホン酸ナトリウムなど)の中に入れて反応させ、表面の電荷を変化させておきます。この方法であれば、常温で、素早く、物質の比重差にも左右されることなく、複合材料を創製できる。しかも吸着させるナノ物質の数なども自在にコントロールできるのです」と武藤教授は語る。
そもそも、どのような物質も、それぞれ固有の電荷を持っている。例えば、シリカ(酸化ケイ素)であれば、中性の水の中に入れればマイナスとなる。しかし、それらは必ずしも均一ではなく、電荷の弱い場合もあり、そのままでは使いづらい。しかし、高分子電解質を使えば、物質に均一に強い電荷を帯びさせることができるのだという。しかも、粒子が元来持つ表面電荷の正負にかかわらず、電荷を自在に変えることもできる。これにより、マトリックス粒子と吸着させるナノ物質の組み合わせ次第で、さまざまな機能を持たせた機能性微粒子の創製が可能になった。
「じつは静電相互作用というのは、機能性「ナノ」薄膜(二次元構造)をつくる際に使われていた手法です。それを我々は粒子などの三次元構造に応用し、集積化に用いることで、さまざまなナノ集合複合体をつくることに成功しました。乾かせばパウダー状にもなるし、濃度を上げてペースト状にすることも、粉末治金といって、プレスして成形して焼き固めて塊にすることもできる。まさに自在に材料をデザインできる点が大きな特長です」と、武藤教授は自負する。
こうしてできた複合粒子は、非常に強い力で結びついているため、安定した材料となる。しかも、機械で混ぜる方式に比べると、エネルギー効率もいい。もっとも、溶液を使うことから濡れ性のない物質や水に浮く物質、水に溶ける物質の創製には不向きだが、さまざまな工夫により、現在ではほぼすべての材料を扱うことができるようになりつつあるという。
その一例として、武藤教授はカーボンナノチューブを用いた導電性材料の開発に取り組んだ。これにより、カーボンナノチューブの添加量を通常よりも大幅に減らし、かつ、きわめて透明度の高い物質をつくることに成功したのである。
量産化に向け、製造プロセスの開発に取り組む
その研究の優位性から、2009〜2012年には、NEDO(独立行政法人新エネルギー・産業技術総合開発機構)の産業技術研究助成事業として採択されたほか、2015年からは内閣府のSIP(戦略的イノベーション創造プログラム)の革新的設計生産技術のプロジェクトの中で研究を深化させている。現在は、複合ナノ材料(複合粒子)を連続的に大量に生産するためのプロセス開発に取り組む。一方で、さまざまな分野の企業からの引き合いも来ているという。
「材料研究というのは、一見、地味な分野ではありますが、材料の特性を変えることで、強度を倍にして半分の薄さにできるといったように、社会に大きなインパクトを与えることができるのです。それだけにやりがいもあります。物質により違いはありますが、数年後には、時間あたり10kg程度の複合粒子をつくれるようにしたいですね」と、武藤教授は抱負を語る。今後、この武藤教授が開発した手法により、どのような夢の材料が開発されるのか、大いに楽しみだ。
取材・文=田井中麻都佳
取材後記
豊橋技術科学大学は、高専との連携を進めていることから高専出身者が多い。武藤教授ご自身も、福島高専のご出身だという。
「高専出身者は、実験に慣れていて、楽しんで取り組む人が多い。だから、何でも実際に手を動かして試してみようとするんですね。現在の研究も、実験してみたら思いのほかうまくいったというのが、最初のスタートだったんですよ」と、武藤教授は笑う。
もともと材料の破壊力学の研究を手掛けていたという武藤教授。評価するセラミックスを自らつくり始めたことが、現在の研究へとつながった。まさに、実験を厭わない姿勢が、大きな研究成果を引き寄せたと言えるだろう。
Researcher Profile

Dr. Hiroyuki Muto studied at Toyohashi Tech, and received his PhD. in 1997, Japan. Since 1997, Dr. Muto has been involved in researching the deformation and flow mechanism of brittle ceramic materials at elevated temperatures at Toyohashi Tech. He was involved in the development of nanocomposite ceramic materials. He was also a visiting research associate of Professor David Willkinson’s group in the Department of Materials Science and Engineering at McMaster University from 2005 to 2006. Currently, Dr. Muto is a professor in the Institute of Liberal Arts and Sciences at Toyohashi University of Technology, Japan. He has more than 15 years of research experience in materials science, and has published extensively in peer-reviewed journals.
Reporter Profile

Madoka Tainaka is a freelance editor, writer and interpreter. She graduated from the Department of Law, at Chuo University, Japan. She served as a chief editor of “Nature Interface” magazine, and on the committee for the Promotion of Information and Science Technology at MEXT (Ministry of Education, Culture, Sports, Science and Technology).
ここでコンテンツ終わりです。
