
ここからコンテンツです。

Garnet-type fast ionic conductor for all-solid-state lithium battery
Development of garnet-type fast ionic conducting oxide for use in all-solid-state lithium batteries By Ryoji Inada
Ryoji Inada and his colleagues have developed a garnet-type, fast ionic conducting oxide. The developed garnet-type oxide showed not only high ionic conductivity, around 1 mS/cm at room temperature, but also high electrochemical stability. Using this material as a solid electrolyte, an all-solid-state lithium battery was fabricated and its reversible charge and discharge reaction was demonstrated successfully. This finding contributes to the realization of highly safe, rechargeable batteries for large-scale power sources.
Rechargeable all-solid-state lithium batteries are expected to be one of the next-generation energy storage devices because of their high energy density, safety, and excellent cycle stability. The materials used for the solid electrolyte must not only have a high lithium-ion conductivity (above 1 mS/cm at room temperature), but also possess chemical stability.
Oxide-based solid electrolyte materials have several advantages over sulfide-based ones, such as their chemical stability and ease of handling. On the other hand, there are certain challenges to overcome if one is aiming at a better electrochemical performance in solid-state batteries with an oxide-based SE, such as the formation of a solid-solid interface with low resistance between the solid electrolyte and the electrode.
In this study, Ryoji Inada and his colleagues at the Department of Electrical and Electronic Information Engineering, developed a garnet-type, fast ionic conducting oxide as the solid electrolyte for an all-solid-state battery. Using this material, a rechargeable all-solid-state battery was fabricated and tested.
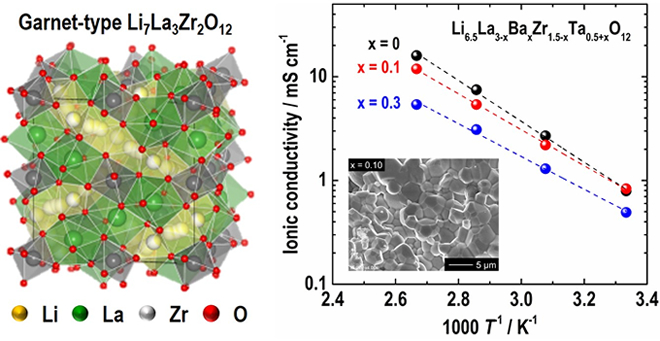
The research team investigated the influence of alien cation (Ba2+ and Ta5+) substitution in Li7La3Zr2O12 (LLZO, Fig. 1) on the crystal phase, microstructure, and ionic conducting property systematically. In order to stabilize the highly conductive cubic garnet phase, the Li concentration in the chemical formulae was fixed at 6.5 so that the formula of the compound may be expressed as Li6.5La3-xBaxZr1.5-xTa0.5+xO12 (LLBZTO).
As a result, the highest room temperature conductivity of 0.83 mS/cm was obtained in the LLBZTO garnet with Ba and Ta contents of 0.1 and 1.6, respectively (Fig. 1). The Activation energy of the LLBZT garnet tended to decrease monotonically with an increasing Ba substitution level; however, the excess Ba and Ta substitution degraded the conductivity.
In addition, they confirmed that the LLBZTO garnet has a wide potential for electrochemical applications, hence, various positive and negative electrode materials can potentially be utilized to construct an all-solid-state battery. We fabricated a TiNb2O7 (TNO) film electrode on LLBZTO using the aerosol deposition method and demonstrated its charge and discharge reaction using a TNO/LLBZTO /Li all-solid-state battery sample (Fig. 2).
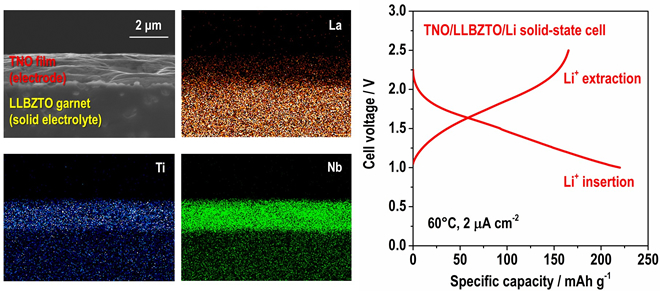
These results indicate that the developed LLBZTO garnet can be used as a solid electrolyte in an all-solid-state battery and contribute to the realization of a very safe rechargeable battery for large-scale power sources, although additional investigation is required to enhance the performance of solid-state batteries. The researchers carried out further study to realize solid-state batteries with high energy density.
The above research results were reported in Frontiers in Energy Research on July 20, 2016.
This work was partly supported by Grants-in-Aid for Challenging Exploratory Research (Grant No. 26630111) and Scientific Research (C) (Grant No. 16K06218) from the Japan Society for the Promotion of Science (JSPS).
Reference
Ryoji Inada, Satoshi Yasuda, Masaru Tojo, Keiji Tsuritani, Tomohiro Tojo and Yoji Sakurai (2016). Development of lithium stuffed garnet-type oxide solid electrolytes with high ionic conductivity for application to all-solid-state batteries, Frontiers in Energy Research 4:28.
DOI:10.3389/fenrg.2016.00028
全固体電池用ガーネット型酸化物イオン伝導体の開発
稲田亮史准教授らの研究グループは,優れた特性を有するガーネット型酸化物イオン伝導体を開発しました。開発した酸化物イオン伝導体は,室温下にて1 mS/cm程度の高いイオン伝導率と優れた電気化学的安定性を備えています。また、本材料を固体電解質として試作した全固体電池にて,可逆的な充放電動作を確認することに成功しました。この結果は,大型電源への応用に適した安全性の高い蓄電池の実現に役立つものです。
充放電可能な全固体リチウム電池は,高いエネルギー密度と安定性・信頼性を同時達成可能な次世代型蓄電池として期待されています。全固体電池用固体電解質として使用される材料には,高いイオン伝導率に加えて優れた化学的安定性が求められます。
酸化物系固体電解質は硫化物系固体電解質と比較して、化学的安定性やハンドリングの容易さの観点で優位です。一方で,優れた電池特性を得るために必要不可欠な電極材料と酸化物固体電解質の接合界面の構築が実用に向けた大きな課題として残されています。
電気・電子情報工学系の稲田亮史准教授らの研究グループは,全固体リチウム電池への応用が期待できるガーネット型酸化物リチウムイオン伝導体を開発しました。さらに、開発材料を固体電解質とした全固体リチウム電池を試作し,その充放電特性を評価しました。
研究グループは,母材料であるLi7La3Zr2O12(リチウム・ランタン・ジルコニウム・酸素,LLZO,図1)に複数の異元素(バリウムBa,タンタルTa)を同時置換した際の結晶相,微細組織およびイオン伝導特性への影響を系統的に調査しました。高いイオン伝導特性を示す立方晶ガーネット構造を安定化するために,分子式中のリチウム量は6.5に固定しました。このため,材料組成は
Li6.5La3-xBaxZr1.5-xTa0.5+xO12 (LLBZTO) で表されます。
結果として,Ba, Ta置換量を各々0.1, 1.6としたLLBZTOガーネットにおいて,最も高い室温イオン伝導率0.83 mS/cmが得られました(図1)。イオン伝導率の活性化エネルギーはBa置換量の増加と共に単調減少する傾向が見られましたが,過剰なBa置換は伝導率の低下を引き起こすことを見出しました。
加えて,同研究グループは,開発したLLBZTOガーネットがリチウム電極基準で0-6 Vと広い電位窓(※1)を有し,全固体電池を構成する際に,様々な正極・負極材料との組み合わせが可能であることを確認しました。チタン-ニオブ複酸化物TiNb2O7(TNO)薄膜電極をLLBZTOガーネット上に形成し,対極を金属リチウムとして試作したTNO/LLBZTO/Li全固体電池にて,可逆的な充放電反応を確認することに成功しました(図2)。
本研究の成果は,開発したLLBZTOガーネットが全固体電池用固体電解質として適用可能であることを示しており,大型電源への応用に適した安全性の高い蓄電池の実現に役立つものです。実用化に向けては更なる電池性能の向上が必要不可欠で、現在同研究グループは全固体電池のエネルギー密度向上に向けた様々な検討を進めています。
本研究成果は、平成28年7月20日(水)にFrontiers in Energy Research誌上に掲載されました。
本研究の一部は日本学術振興会(JSPS)科学研究費助成事業(課題番号26630111および16K06218)の支援の下で実施されたものです。
Researcher Profile

| Name | Ryoji Inada |
|---|---|
| Affiliation | Department of Electrical and Electronic Information Engineering |
| Title | Associate Professor |
| Fields of Research | Energy Conversion Engineering / Electrical and Electronic Material Engineering / Non-Destructive Testing |
ここでコンテンツ終わりです。
